Thermo Scientific™ Hypericin from Hypericum perforatum, 98%
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | cell Signalling, Apoptosis | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Biochemical Reagent | Forms | Solid |
| Product Brand | Thermo Fisher Scientific™, thermo Scientific | ||
| Product Grade | Molecular Biology | Formula | C₃₀H₁₆O₈ |
Thermo Scientific™ Hypericin (≥98%) is a naturally occurring anthraquinone derivative extracted from Hypericum perforatum (St. John’s wort). It exhibits a diverse range of pharmacological and biochemical activities, including antidepressant, antiviral, antineoplastic, and immunostimulatory effects.
As a potent inhibitor of protein kinase C (PKC), hypericin induces photosensitized apoptosis, making it an ideal agent for photodynamic therapy (PDT) research. It also acts on multiple neurotransmitter pathways, inhibiting neuronal uptake of serotonin, dopamine, norepinephrine, GABA, and glutamate, thereby contributing to its antidepressant and neuromodulatory properties.
Key Features
- High Purity: ≥97.5% (assay by supplier’s CoA).
- Multifunctional Agent: Exhibits antiviral, antitumor, antidepressant, and phototoxic activities.
- Protein Kinase C Inhibitor: Induces apoptosis via light-activated PKC inhibition.
- Photosensitizer for PDT: Generates reactive oxygen species upon irradiation.
- Antiviral Mechanism: Inhibits virus assembly, shedding, and replication in infected cells.
- Research-Grade Quality: Suitable for biochemical, pharmacological, and photodynamic research.
- Form: Dark brown to black powder.
- Research Use Only (RUO): Not for diagnostic or therapeutic use.
Chemical Identifiers
| Property | Value |
|---|---|
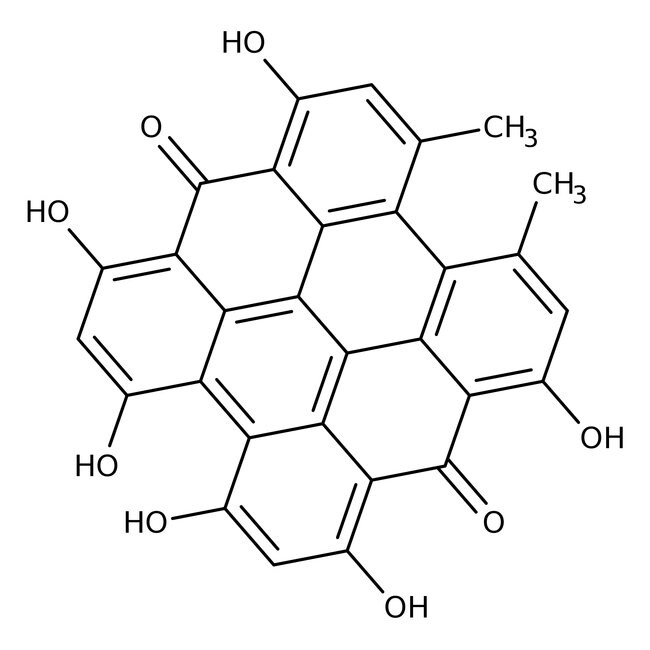
| Chemical Name (IUPAC) | 7,11,13,16,18,22-hexahydroxy-5,24-dimethyloctacyclo[13.11.1.1²,¹⁰.0³,⁸.0⁴,²⁵.0¹⁹,²⁷.0²¹,²⁶.0¹⁴,²⁸]octacosa-1(27),2(28),3,5,7,10,12,14,16,18,21,23,25-tridecaene-9,20-dione |
| Synonyms | Hypericin, Hypericine, Hypericum Red, Hipericina, St. John’s Wort pigment |
| CAS Number | 548-04-9 |
| Molecular Formula | C₃₀H₁₆O₈ |
| Molecular Weight | 504.45 g/mol |
| InChI Key | BTXNYTINYBABQR-UHFFFAOYSA-N |
| SMILES | CC1=CC(O)=C2C(=O)C3=C(O)C=C(O)C4=C5C(O)=CC(O)=C6C(=O)C7=C(O)C=C(C)C8=C7C(=C56)C(C2=C18)=C34 |
Specifications
| Parameter | Specification |
|---|---|
| Purity (HPLC) | ≥97.5% |
| Form | Powder |
| Appearance | Dark brown to black |
| Mechanism | Inhibits protein kinase C; induces photosensitized apoptosis |
| Solubility | Slightly soluble in water; soluble in ethanol, DMSO, and methanol |
| Storage Conditions | Store in a cool, dry place away from light |
| Stability | Light-sensitive; handle under subdued lighting |
| Packaging | Glass bottle |
| Intended Use | Research Use Only (RUO) |
Applications
- Photodynamic Therapy (PDT): Used as a light-activated photosensitizer for anticancer and antiviral research.
- Antiviral Studies: Investigations of viral replication inhibition and encapsulated virus suppression.
- Cancer Research: Evaluation of apoptosis and ROS-mediated cytotoxicity in tumor cells.
- Neurobiology: Studies of antidepressant mechanisms and neurotransmitter uptake inhibition.
- Molecular Biology: PKC inhibition and signal transduction pathway analysis.
- Fluorescence and Imaging Studies: Exploited for its photophysical and fluorescence properties.
References
- Biochem. Biophys. Res. Commun. (1989), 165, 1207 – Inhibition of Protein Kinase C.
- Photochem. Photobiol. (1991), 54, 95 – Antiviral and Antitumor Photosensitizer.
- Kusari, S. et al. “Spatial chemo-profiling of hypericin and related phytochemicals in Hypericum species.” Anal. Bioanal. Chem. (2015) 407(16), 4779–4791.
- Vogler, A. “Fluorescence spectra of complexes of hypericin with s² metal cations.” Inorg. Chem. Commun. (2015) 54, 61–62.
Safety & Handling
- Signal Word: Warning
-
Hazard Statements:
- H302 – Harmful if swallowed
- H317 – May cause an allergic skin reaction
-
Precautionary Statements:
- P280 – Wear protective gloves/protective clothing
- P302 + P352 – If on skin: Wash with plenty of water
- P312 – Call a doctor if unwell
- Classification: Acute Oral Toxicity Cat. 4 | Skin Sensitization Cat. 1
The Thermo Scientific™ Hypericin (98%) is a high-purity anthraquinone derivative with potent antiviral, anticancer, and photodynamic properties.
It acts as a photosensitizer, protein kinase C inhibitor, and antidepressant research compound, making it a versatile reagent for neuropharmacology, oncology, and phototherapy studies.
Manufactured to Thermo Scientific’s quality standards, it ensures reliable and reproducible performance in biochemical and photobiological research applications.
- Pack Size: 25 mg 100 mg




 0
0