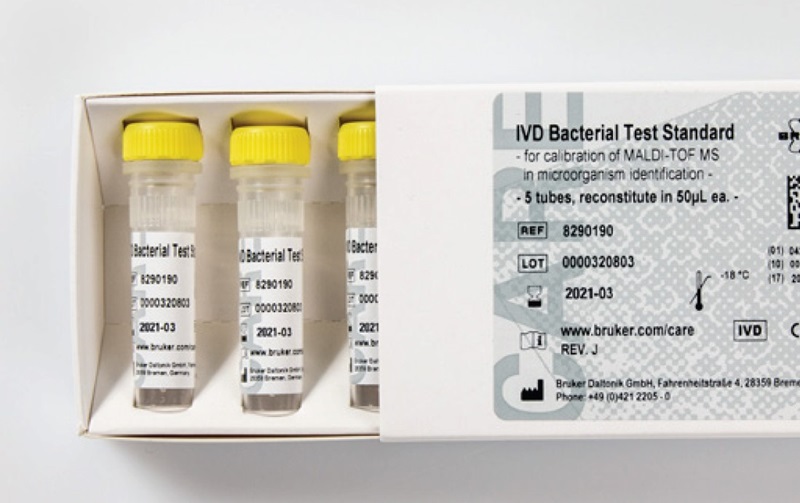
MALDI Biotyper® IVD Bacterial Test Standard, 50 μL per tube
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | MALDI-MS | ||
| Storage Temperature | -20°C | ||
| Product Type | Calibration Standard | Forms | Liquid |
| Product Brand | Bruker | ||
| Product Grade | Microbiology grade | ||
The MALDI Biotyper® IVD Bacterial Test Standard (BTS) is a quality control reagent designed specifically for use with the MALDI Biotyper® IVD system. This standard ensures the accuracy and reliability of microbial identification via Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS). It is an integral component in clinical microbiology for routine verification of instrument performance and the identification of bacterial species.
Key Features
- Certified Quality Control Standard:
- Designed for the MALDI Biotyper® IVD system for clinical use.
- Ensures compliance with stringent in vitro diagnostic (IVD) standards.
- Comprehensive Application:
- Prepares and validates bacterial reference spectra for microbial identification.
- Ensures accuracy and reproducibility of MALDI-TOF MS results.
- Stable and Ready-to-Use:
- Provided in a lyophilized format for long-term stability.
- Easy reconstitution process for daily use.
- Wide Range of Organisms:
- Includes a defined bacterial mix with a reproducible protein profile.
- Supports identification across a wide range of clinically significant bacteria.
- Traceability and Compliance:
- Manufactured under rigorous ISO 13485 standards for quality.
- Fully traceable lot-specific documentation.
Technical Specifications
- Format: Lyophilized bacterial reference material.
- Storage: Stable at -20°C.
- Use: Reconstitution with a buffer for application in MALDI-TOF MS calibration.
- Certified Quality: Validated for clinical diagnostic workflows.
Use Cases
- Routine Calibration of MALDI Biotyper®:
- Ensures system accuracy and alignment before microbial identification runs.
- Ideal for daily quality control in diagnostic laboratories.
- Quality Control for Clinical Microbiology Labs:
- Ensures consistency in the identification of bacterial species.
- Essential for accreditation compliance in clinical settings.
- Validation of New MALDI-TOF Systems:
- Used during installation to validate MALDI Biotyper® systems.
- Performance Verification:
- Checks the instrument's sensitivity and resolution for identifying complex bacterial profiles.
- Training and Proficiency Testing:
- Provides a consistent standard for lab personnel training.
- Useful for proficiency testing to ensure operator and system competency.
Applications
- Clinical Microbiology:
- Identification of pathogenic bacteria from patient samples (e.g., blood, urine, sputum).
- Facilitates antimicrobial stewardship programs by quickly identifying pathogens.
- Pharmaceutical Testing:
- Ensures quality and sterility in pharmaceutical production by detecting contaminants.
- Food and Environmental Microbiology:
- Identifies bacterial contaminants in food and water safety testing.
- Research Applications:
- Supports microbiome analysis and bacterial profiling in academic studies.
Benefits
- Accuracy and Reliability:
- Ensures high-confidence identification of bacterial species.
- Ease of Use:
- Pre-prepared standard reduces preparation time and operator error.
- Cost-Effective:
- Regular use prolongs instrument life by ensuring proper calibration.
- Regulatory Compliance:
- Supports clinical lab adherence to regulatory standards for diagnostics.
Conclusion
The MALDI Biotyper® IVD Bacterial Test Standard (BTS) is a critical component in microbiological diagnostics, ensuring consistent, accurate, and reproducible identification of bacteria. Its applications span across clinical, pharmaceutical, food safety, and research sectors, supporting quality assurance and advancing microbial diagnostics.




 0
0