Thermo Scientific™ Sensititre™ Mycobacterium tuberculosis MYCOTBI AST Plate
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | 2-8°C |
| Product Type | AST Panel |
| Product Brand | Thermo Fisher Scientific™ |
| Product Grade | Microbiology grade |
Comprehensive MIC-Based Drug Susceptibility Testing for Tuberculosis
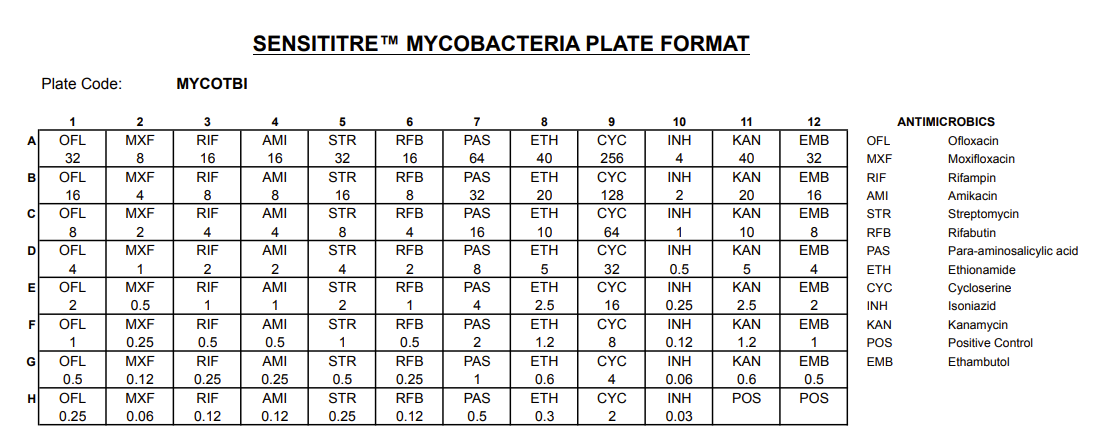
The Thermo Scientific™ Sensititre™ MYCOTBI Plate is a cost-effective and comprehensive antimicrobial susceptibility testing (AST) solution designed for Mycobacterium tuberculosis. This ready-to-use, 96-well MIC plate includes 12 first-line and second-line anti-tuberculosis drugs in a single microbroth dilution format, enabling laboratories to confidently determine the minimum inhibitory concentration (MIC) values critical for guiding effective TB therapy.
With an extended shelf life, room-temperature storage, and compatibility with both manual and digital reading platforms (e.g., Sensititre™ Vizion™), the MYCOTBI plate simplifies workflow while maintaining clinical reliability in TB drug resistance detection.
Key Features & Benefits
-
Complete TB Drug Panel
Contains 12 antimicrobials used in both first-line and second-line tuberculosis treatment
Ideal for identifying multidrug-resistant (MDR-TB) and extensively drug-resistant (XDR-TB) strains -
Accurate MIC-Based Methodology
Based on the broth microdilution (BMD) gold standard for AST
Delivers precise, reproducible MIC values for each drug tested -
Extended Shelf Life & Easy Storage
18–24 months shelf life
Room-temperature storage eliminates the need for cold chain logistics -
Flexible Reading Options
Can be read manually or using the Sensititre™ Vizion™ Digital MIC Viewing System
Compatible with SWIN™ Software for result recording, analysis, and LIS connectivity -
Quality Control Assurance
Includes on-scale QC ranges for immediate validation of plate and test performance -
Efficient, Low-Waste Design
Individually packaged plates allow one plate per test, reducing waste and cost
Applications
- Clinical Microbiology Labs: Perform MIC testing of Mycobacterium tuberculosis isolates
- Public Health Labs: Surveillance of MDR-TB and XDR-TB prevalence
- Reference Laboratories: Confirmatory susceptibility testing
- TB Treatment Programs: Support for regimen selection and drug resistance management
Specifications Table
| Attribute | Details |
|---|---|
| Product Name | Sensititre™ MYCOTBI MIC Plate |
| Catalog Number | MYCOTBI |
| Quantity per Pack | 10 plates |
| Antimicrobials Included | First-line and second-line TB drugs (12 total) |
| Broth Type | Middlebrook 7H9 with OADC (Part No. T3440) |
| Inoculum Preparation | 0.5 McFarland using Saline Tween + Glass Beads (T3490) |
| Transfer Volume / Well | 100 µL |
| Incubation Time & Conditions | 10–21 days at 35–37°C in ambient air (O₂) |
| Reading Method | Manual or Sensititre™ Vizion™ System |
| Species Tested | Mycobacterium tuberculosis |
| Test Type | Minimum Inhibitory Concentration (MIC) |
| Isolates per Plate | 1 isolate per plate |
| Storage Conditions | Room temperature |
| Shelf Life | 18–24 months |
| Packaging | Box of 10 plates |
| Intended Use | In vitro diagnostic use, professional use only |
| Regulatory Notes | Not automated; requires pure, agar-grown cultures |
The Thermo Scientific™ Sensititre™ MYCOTBI AST Plate is a powerful, MIC-based solution for laboratories testing Mycobacterium tuberculosis against first and second-line drugs. With its user-friendly format, long shelf life, and compatibility with existing Sensititre™ systems, it provides dependable, actionable results that support clinicians in tailoring therapy for TB patients—especially in the context of drug-resistant strains.




 0
0