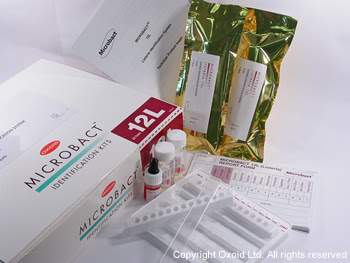
Thermo Scientific™ Oxoid™ Microbact™ Listeria 12L Kit, 20 Tests
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Microbial ID System | Forms | Kit with Various components |
| Product Brand | Thermo Fisher Scientific™, Oxoid | ||
| Product Grade | Microbiology grade | ||
The Oxoid™ Microbact™ Listeria 12L Kit is a comprehensive, biochemical-based identification system designed for the rapid and reliable identification of Listeria species, including Listeria monocytogenes, the primary pathogen responsible for human listeriosis. This kit is ideal for clinical, food, and environmental laboratories, enabling efficient workflows for detecting and enumerating Listeria spp.
Key Features
- Fast and Efficient:
- Provides accurate identification of Listeria spp. in as little as 4 hours.
- Comprehensive Testing:
- Incorporates 12 analytes:
- 11 carbohydrate utilization tests.
- 1 rapid microhaemolysis test.
- Supports the definitive identification of all Listeria spp., including L. monocytogenes and L. innocua.
- Incorporates 12 analytes:
- Ease of Use:
- No need for:
- Subculturing on blood agar.
- CAMP tests.
- Developing reagents.
- Results are visually interpreted using the Microbact Identification Package (MB1244A).
- No need for:
- Reliable and Flexible:
- Compatible with chromogenic and selective media.
- Designed for both clinical and food laboratory applications.
- Substrates meet international standard methods, including FDA BAM and ISO.
Specifications
| Property | Details |
|---|---|
| Description | Microbact Listeria 12L Kit |
| Detectable Analytes | Listeria species |
| Quantity | 20 Tests/Kit |
| Packaging Type | 1 Box |
| Includes | 20 test strips, suspending medium vials (3mL), holding tray, technical product insert, and report forms |
| Additional Items Required | Microbact Software Program (MB1244A), Haemolysin Reagent (MB1249A) |
Applications
- Clinical Microbiology:
- Identifies Listeria monocytogenes and other Listeria spp., aiding in the diagnosis of severe infections like:
- Septicemia.
- Meningitis.
- Encephalitis.
- Intrauterine infections in pregnant women.
- Identifies Listeria monocytogenes and other Listeria spp., aiding in the diagnosis of severe infections like:
- Food Safety Testing:
- Detects Listeria spp. in food products, including processed and ready-to-eat foods.
- Validated for use in food and environmental testing workflows.
- Environmental Monitoring:
- Identifies Listeria contamination in environmental samples from food production and processing facilities.
Advantages
- Rapid Results: Delivers reliable identification within hours, enabling quick decision-making.
- User-Friendly: Requires minimal preparation and provides clear, easily interpretable results.
- Versatility: Suitable for a variety of laboratory settings and sample types.
- Validated Methods: Substrates conform to FDA BAM and ISO standards, ensuring accuracy and compliance.
Workflow Overview
- Preparation:
- Follow the kit instructions to prepare samples and test strips.
- Incubation:
- Analyze carbohydrate utilization and haemolysis patterns.
- Interpretation:
- Use the Microbact Identification Package (MB1244A) to interpret distinct color changes and settling patterns.
- Reporting:
- Record results using the included report forms.
Additional Information
- Catalog Number: MB1128A.
- Packaging: Includes all necessary materials for 20 tests, excluding additional software or reagents.
- Intended Use: For identifying Listeria species based on biochemical substrate utilization and haemolysin production.
- Software Updates: Thermo Scientific provides downloadable updates for the Microbact Software Program via their Biochemical Identification Software portal.
Why Choose the Oxoid Microbact Listeria 12L Kit?
- Accurate and reliable identification of Listeria spp.
- Simplified workflow requiring no complex testing reagents.
- Fully self-contained kit, minimizing the need for additional resources.
- Supports compliance with international standards in food safety and clinical diagnostics.




 0
0