Specifications:
| Application | Microbial Identification/AST |
| Storage Temperature | 2-8°C |
| Product Type | Microbial ID System |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Rapid Presumptive Identification of Listeria monocytogenes and Group B Streptococci via Enhanced Hemolysis
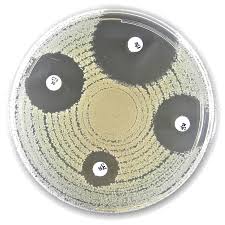
The Liofilchem® CAMP Test is a ready-to-use diagnostic tool for detecting the CAMP factor—a hemolytic protein produced by Group B streptococci and some species of Listeria. The test enhances beta-hemolysis in the presence of Staphylococcus aureus or Rhodococcus equi, enabling rapid and visual confirmation of CAMP-positive organisms through a synergistic hemolytic reaction.
The discs are impregnated with viable, stabilized bacterial strains obtained from the American Type Culture Collection (ATCC) and are available in two formulations:
- 88021 – with Staphylococcus aureus ATCC 25923
- 88027 – with Rhodococcus equi ATCC 6939
The CAMP Test-R is widely used in clinical microbiology and food safety laboratories for the differentiation of Listeria monocytogenes from other Listeria species and for confirming Group B Streptococcus identification.
Key Features & Benefits
- ✅ Rapid and Easy-to-Use – Visual interpretation after 24-hour incubation
- ✅ Standardized Control Organisms – Discs impregnated with ATCC strains
- ✅ Reliable Differentiation – Detects enhanced hemolysis for identification of CAMP-positive strains
- ✅ Non-hazardous – Does not contain substances classified as dangerous under current legislation
- ✅ Versatile Application – Effective in both clinical and food microbiology testing
- ✅ Stability Verified – Maintains performance even after temporary storage at room temperature or short-term heat exposure
- ✅ In Vitro Diagnostic Use – Designed for professional microbiology laboratories
Product Specifications
| Attribute | Details |
|---|---|
| Product Name | CAMP Test |
| Catalog Numbers | 88021 (S. aureus ATCC 25923), 88027 (R. equi ATCC 6939) |
| Pack Size | 30 Tests |
| Detection Target | CAMP factor (pyrrolidonyl-dependent hemolysis enhancer) |
| Test Method | Enhanced hemolysis on Tryptic Soy Agar + 5% Sheep Blood |
| Result Time | 24 hours at 36 ± 1 °C |
| Readout | Purple zone of enhanced hemolysis = Positive test |
| Storage Conditions | 2–8 °C (Stable for short-term transport at 18–25 °C for 4 days or 35–39 °C for 48 hours) |
| Shelf Life | See product label |
| Safety | Non-hazardous; refer to SDS for good laboratory practice |
| Disposal | Dispose in accordance with national/local regulations |
Technique (Test Protocol)
- Place a CAMP Test disc on a Tryptic Soy Agar + 5% Sheep Blood plate.
- Moisten the disc with a drop of sterile saline.
- Using a sterile loop, streak the test organism near the disc without touching it.
- Incubate at 36 ± 1°C for 24 hours.
-
Observe for a hemolytic zone near the disc:
- Positive Test: Enhanced (synergistic) hemolysis near the disc
- Negative Test: No hemolytic enhancement
Interpretation Table: CAMP Reaction for Listeria spp.
| Species | β-Hemolysis | CAMP Test |
|---|---|---|
| Listeria monocytogenes | + | + |
| Listeria innocua | – | – |
| Listeria ivanovii | + | – |
| Listeria seeligeri | + | + |
| Listeria welshimeri, L. grayi spp. | – | – |
| Jonesia denitrificans | – | – |
Applications
🧫 Clinical Diagnostics – Identification of Group B Streptococci and Listeria monocytogenes
🥩 Food Safety Testing – Screening of dairy, meat, and ready-to-eat food for Listeria monocytogenes
🔬 Microbiology Research – Study CAMP factor and interspecies hemolytic interactions
📋 ISO-Compliant Testing – Aligned with ISO 11290-1:1996 for food microbiology
Ordering Information
| Catalog # | Strain Impregnation | Pack Size |
|---|---|---|
| 88021 | Staphylococcus aureus ATCC 25923 | 30 Tests |
| 88027 | Rhodococcus equi ATCC 6939 | 30 Tests |
The Liofilchem® CAMP Test provides a simple, accurate method for presumptive identification of Listeria monocytogenes and Group B Streptococci based on the CAMP phenomenon. With standardized ATCC strains, reliable hemolytic reaction, and ease of use, CAMP Test-R is a dependable choice for laboratories conducting routine microbial identification in clinical and food safety settings.
- variant: Impregnated with Staphylococcus aureus ATCC 25923 (CAMP Test-S) Impregnated with Rhodococcus equi ATCC 6939 (CAMP Test-R)




 0
0