Sigma-Aldrich Heparin sodium, BRP, European Pharmacopoeia (EP) Reference Standard, 1.1 mL
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Analytical Chemistry | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Calibration Standard | Forms | Liquid |
| Product Brand | Sigma-Aldrich | ||
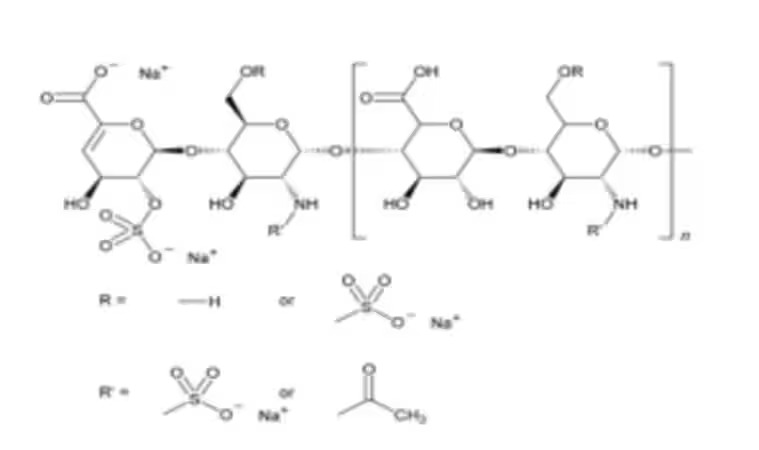
| Product Grade | Analytical grade | Formula | (C₁₂H₁₆NS₂Na₃)₂₀ |
Sigma-Aldrich Heparin Sodium BRP is a certified European Pharmacopoeia (EP) reference standard, issued and maintained by the European Directorate for the Quality of Medicines (EDQM). It is used for official compendial testing and validation procedures, especially in the analysis of unfractionated heparin (UFH).
This liquid standard is supplied in a 1.1 mL neat format and includes assigned biological activities to ensure accuracy and traceability in pharmacopoeial assays.
Key Features and Benefits
- EP-authorized reference substance for heparin testing
-
Assigned bioactivity for:
- Anti-IIa activity: 985 IU/mL
- Anti-Xa activity: 995 IU/mL
- Clotting assay (sheep plasma): 1035 IU/mL
- Compliant with European Pharmacopoeia monographs
- Neat, ready-to-use format with validated potency
- Suitable only for compendial methods as specified by Ph. Eur.
Applications
This reference standard is validated for use in the following European Pharmacopoeia analytical procedures:
-
Ph. Eur. General Text 2.7.5 – Assay of Unfractionated Heparin
- Determination of anti-IIa and anti-Xa activity
-
Ph. Eur. Monograph 0878 – Test for Heparin in Human Antithrombin III
- Using a sheep plasma clotting assay
-
Ph. Eur. Monograph 0569 – Assay of Protamine Sulfate
- Standard for evaluating protamine's heparin-neutralizing capacity
This material is not intended for human or veterinary therapeutic use and must only be used as prescribed by the pharmacopoeia.
Specifications
| Attribute | Details |
|---|---|
| CAS Number | 9041-08-1 |
| EDQM Catalog Number | H0200000 |
| Format | Neat (liquid) |
| Pack Size | 1.1 mL |
| Assigned Activities | 985 IU/mL (anti-IIa), 995 IU/mL (anti-Xa), 1035 IU/mL (clotting assay) |
| Grade | Pharmaceutical Primary Standard |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| SMILES / InChI | Provided for chemical identity only |
| Manufacturer | EDQM (via Sigma-Aldrich) |
This product is provided exactly as delivered by the EDQM. All regulatory, safety, and test information is issued and maintained by the Pharmacopoeia authority. Suitability for any use outside official Ph. Eur. procedures is not guaranteed and is the user's responsibility.




 0
0