Thermo Scientific™ Oxoid™ Trimethoprim/Sulfamethoxazole SXT 1:19 Antimicrobial Susceptibility discs, 25µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Thermo Fisher Scientific™, Oxoid |
| Product Grade | Microbiology grade |
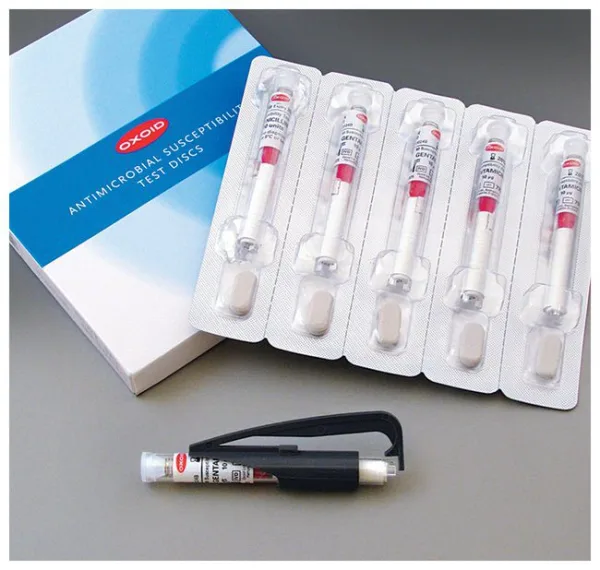
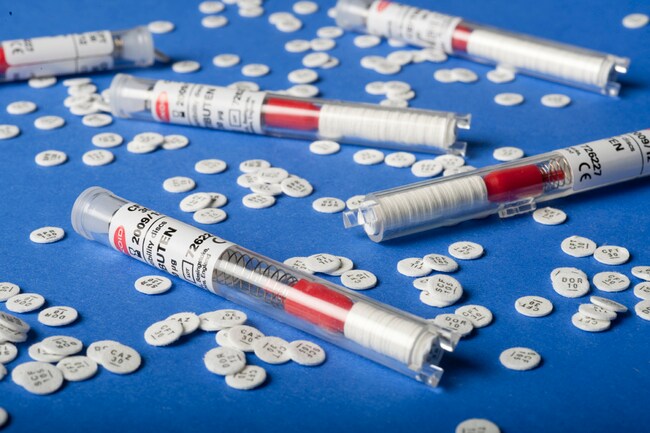
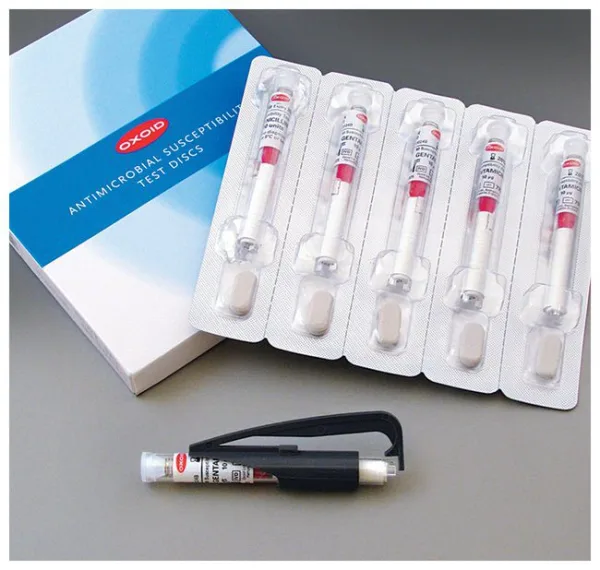
Thermo Scientific™ Oxoid™ Trimethoprim/Sulfamethoxazole 1:19 Antimicrobial Susceptibility Discs are precision-manufactured, cartridge-based paper discs used in manual disk diffusion testing to determine microbial susceptibility to the combination antibiotic trimethoprim and sulfamethoxazole. The disc contains 25 µg of the combined agents in a 1:19 ratio, following CLSI, DIN, and SFM standards.
Ideal for Kirby-Bauer disk diffusion assays, these discs are supplied in spring-loaded, shatterproof cartridges, each individually sealed with a desiccant to maintain <2% moisture and ensure long-term stability.
Key Features and Benefits
- 1:19 Combination Ratio: Reflects clinical dosing and pharmacodynamics of trimethoprim and sulfamethoxazole
- Standardized Concentration: 25 µg per disc for consistency and regulatory compliance
- Broad Spectrum Utility: Effective against a wide range of Gram-positive and Gram-negative pathogens
- Compliance: Manufactured in accordance with CLSI, DIN, and SFM guidelines
- Enhanced Usability: Spring-loaded cartridges allow easy disc dispensing; visible cartridge indicators
- Visual Interpretation: Assesses not only susceptibility but also signs of contamination, mutant populations, synergy, and inoculum density
- Reliable Storage: Individually sealed cartridges ensure reagent stability and reproducibility
Intended Use Statement
These discs are intended for semi-quantitative in vitro susceptibility testing of clinical isolates using the agar diffusion method. They help guide antibiotic therapy selection for infections where Trimethoprim/Sulfamethoxazole is active. To be used with pure agar-grown cultures in clinical laboratories by trained professionals.
Specifications
| Parameter | Value |
|---|---|
| Antibiotic Agent | Trimethoprim/Sulfamethoxazole (1:19) |
| Antibiotic Code | SXT |
| Disc Concentration | 25 µg |
| Format | Paper disc, 6 mm diameter |
| Compliance | CLSI, DIN, SFM |
| Discs per Cartridge | 50 |
| Cartridges per Pack | 5 |
| Total Discs per Pack | 250 |
| Packaging | Moisture-controlled with desiccant (<2% moisture) |
| Product Type | Antimicrobial Susceptibility Discs (AST) |
| Storage Conditions | Room temperature (dry environment) |
| Unit Size | Each pack (5 cartridges) |
| Use Classification | In vitro diagnostic use, professional only |
Applications
- Clinical antimicrobial susceptibility testing
- Infectious disease laboratories
- Routine hospital AST workflows
- Microbial resistance profiling
- Pharmaceutical microbiology and drug screening
- Phenotypic validation for trimethoprim/sulfamethoxazole resistance
Thermo Scientific™ Oxoid™ SXT 25 µg Discs deliver accurate, compliant, and reliable testing results in routine AST for trimethoprim/sulfamethoxazole susceptibility. With quality-controlled manufacturing and convenient cartridge format, these discs are trusted by clinical microbiology labs worldwide for high-throughput and high-precision AST workflows.




 0
0