Specifications:
| Application | Clinical microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Microbial ID System |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
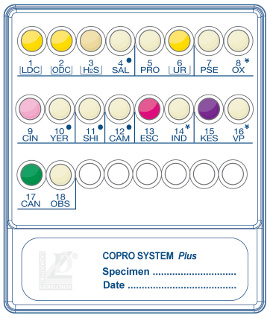
Liofilchem™ COPRO SYSTEM
Direct Microbial Identification of Intestinal Pathogenic Microorganisms
The Liofilchem™ COPRO SYSTEM is a diagnostic tool designed for the rapid and reliable identification of intestinal pathogenic microorganisms directly from fecal specimens. This system combines biochemical and enzymatic tests into a compact panel, enabling the simultaneous identification of multiple pathogens and their antimicrobial susceptibility testing.
Purpose
- Identification and differentiation of pathogenic microorganisms involved in gastrointestinal infections.
- Direct testing of fecal specimens or rectal swabs.
- Rapid results for clinical decision-making.
Target Microorganisms
The system identifies a range of intestinal pathogens, including:
- Pathogenic microorganisms:
- Salmonella spp.
- Shigella spp.
- Campylobacter jejuni
- Escherichia coli O157
- Proteus spp., Pseudomonas spp., Yersinia enterocolitica, and more.
- Flora evaluation:
- Escherichia coli, Enterobacter spp.
- Opportunistic pathogens:
- Klebsiella spp., Candida spp.
Key Features
- Direct Testing:
- Fecal specimens or rectal swabs are directly inoculated, eliminating the need for enrichment steps.
- Comprehensive Results:
- Simultaneous identification of multiple pathogens and antimicrobial susceptibility.
- Rapid Turnaround:
- Results available in 18–24 hours.
- Ease of Use:
- Clear, well-defined color changes in wells for straightforward interpretation.
- Antimicrobial Susceptibility Testing (AST):
- Includes a panel of antimicrobials for assessing resistance patterns.
Test Procedure
1. Sample Collection and Preparation
- Collect fecal specimens or rectal swabs using sterile techniques.
- Transport samples in appropriate containers to the laboratory.
- Prepare a suspension of the specimen in sterile diluent as per protocol.
2. Inoculation
- Using a sterile pipette or loop, inoculate the wells of the COPRO SYSTEM panel with the prepared sample suspension.
- Seal the panel with the provided adhesive film or cover.
3. Incubation
- Place the inoculated panel in an incubator set to 36 ± 1°C.
- Incubate for 18–24 hours under aerobic conditions.
4. Observation
- Observe each well for color changes corresponding to biochemical or enzymatic activity.
- Compare the results with the interpretation chart provided in the system's manual.
5. Identification
- Record the results for each biochemical reaction.
- Generate a biochemical profile code from the observed results.
- Use the Liofilchem™ Code Book or software for pathogen identification.
6. Antimicrobial Susceptibility Testing (AST)
- Assess growth inhibition in wells containing antimicrobial agents.
- Interpret results as:
- Resistant: Growth in the presence of the antimicrobial.
- Intermediate: Partial inhibition of growth.
- Sensitive: No growth observed.
Biochemical and Enzymatic Tests
The COPRO SYSTEM includes tests for:
- Carbohydrate Fermentation: Detects the ability to metabolize sugars like lactose, glucose, and sucrose.
- Enzymatic Reactions: Urease, oxidase, and indole production.
- Hydrogen Sulfide Production: Differentiates Salmonella from Shigella.
- Toxin Detection: Identifies Escherichia coli strains producing Shiga toxins.
- Pathogen-Specific Tests: Targets specific metabolic traits of Yersinia enterocolitica and Campylobacter jejuni.
Antimicrobial Agents in AST
| Antimicrobial | Targeted Pathogens |
|---|---|
| Ciprofloxacin | Salmonella spp., Shigella spp. |
| Ceftriaxone | Broad-spectrum Gram-negative |
| Piperacillin/Tazobactam | Enteric bacteria |
| Amoxicillin/Clavulanic Acid | Escherichia coli, Proteus spp. |
| Trimethoprim/Sulfamethoxazole | Shigella spp., Yersinia spp. |
| Nitrofurantoin | Enterobacteriaceae |
Interpretation of Results
- Positive Reaction: Color change specific to each test well.
- Negative Reaction: No color change.
- Refer to the Interpretation Chart provided with the panel to evaluate the results.
Applications
- Clinical Diagnostics:
- Identification of intestinal pathogens causing gastroenteritis, diarrhea, and foodborne infections.
- Assessment of antimicrobial resistance for targeted therapy.
- Research:
- Studies on intestinal flora and pathogenic mechanisms.
- Public Health Monitoring:
- Surveillance of outbreaks and antibiotic-resistant strains.
Benefits
| Feature | Benefit |
|---|---|
| Direct Testing | Eliminates the need for enrichment steps, saving time and resources. |
| Rapid Results | Results in 18–24 hours, facilitating prompt clinical decisions. |
| Comprehensive Identification | Simultaneous detection of multiple intestinal pathogens. |
| Ease of Use | Clear color changes simplify result interpretation. |
| AST Integration | Provides susceptibility patterns for effective antimicrobial therapy. |
Packaging Information
| Product | Description | Reference | Tests |
|---|---|---|---|
| COPRO SYSTEM | ID and AST for intestinal pathogens | 71650 | 20 tests |
Why Choose Liofilchem™ COPRO SYSTEM?
- Comprehensive Diagnostic Tool: Combines identification and susceptibility testing in a single panel.
- Time-Saving: Direct inoculation and rapid results streamline laboratory workflows.
- Cost-Effective: Reduces the need for multiple separate tests.
- Accurate and Reliable: High-quality biochemical and enzymatic tests ensure precise identification of intestinal pathogens.
The Liofilchem™ COPRO SYSTEM and bioMérieux's API 20E, API 20NE, and API Campy are diagnostic tools designed for the identification of pathogenic microorganisms. Below is a comparative overview of these systems:
| Feature | Liofilchem™ COPRO SYSTEM | API 20E | API 20NE | API Campy |
|---|---|---|---|---|
| Manufacturer | Liofilchem S.r.l. | bioMérieux | bioMérieux | bioMérieux |
| Target Microorganisms | Intestinal pathogens including Salmonella spp., Shigella spp., Campylobacter jejuni, Escherichia coli O157, Proteus spp., Pseudomonas spp., Yersinia enterocolitica, and others. | Enterobacteriaceae and other non-fastidious Gram-negative rods. | Non-fermenting Gram-negative bacteria such as Pseudomonas spp., Acinetobacter spp., Flavobacterium spp., and others. | Campylobacter species, primarily Campylobacter jejuni and Campylobacter coli. |
| Sample Type | Direct testing from fecal specimens or rectal swabs. | Requires isolated bacterial colonies from culture. | Requires isolated bacterial colonies from culture. | Requires isolated bacterial colonies from culture. |
| Number of Tests | 18 biochemical tests. | 21 biochemical tests | 20 biochemical tests. | 20 biochemical tests. |
| Incubation Time | 18–24 hours. | 18–24 hours. | 24–48 hours. | 24 hours. |
| Antimicrobial Susceptibility Testing (AST) | Integrated AST for selected antimicrobials, including Ciprofloxacin, Ceftriaxone, Piperacillin/Tazobactam, Trimethoprim/Sulfamethoxazole, Rifampicin, Neomycin/Bacitracin, and Amoxicillin/Clavulanic Acid. | Not included; separate testing required. | Not included; separate testing required. | Not included; separate testing required. |
| Ease of Use | Direct inoculation from fecal samples simplifies workflow; clear color changes facilitate interpretation. | Requires prior isolation of colonies; interpretation based on color changes and numerical profile analysis. | Requires prior isolation of colonies; interpretation based on color changes and numerical profile analysis. | Requires prior isolation of colonies; interpretation based on color changes and numerical profile analysis. |
| Automation Compatibility | Compatible with automatic reading systems for increased efficiency. | Primarily manual, though compatible with certain automation tools. | Primarily manual, though compatible with certain automation tools. | Primarily manual, though compatible with certain automation tools. |
| Cost-Effectiveness | Cost-effective solution for clinical laboratories. | Generally more expensive due to proprietary materials and established branding. | Generally more expensive due to proprietary materials and established branding. | Generally more expensive due to proprietary materials and established branding. |
Key Differences:
- Sample Processing: The COPRO SYSTEM allows for direct testing from fecal specimens, streamlining the diagnostic process. In contrast, the API systems require prior isolation of bacterial colonies, which can extend the time to results.
- Antimicrobial Susceptibility Testing: The COPRO SYSTEM integrates AST within the same panel, providing both identification and susceptibility profiles simultaneously. The API systems do not include AST, necessitating separate testing procedures.
- Target Organisms: While there is overlap, the COPRO SYSTEM is specifically designed for a broad range of intestinal pathogens, including Campylobacter jejuni and Escherichia coli O157, which are not the primary focus of the API 20E and API 20NE systems.
In summary, the Liofilchem™ COPRO SYSTEM offers a more streamlined and comprehensive approach for the direct identification and susceptibility testing of intestinal pathogens compared to the API 20E, API 20NE, and API Campy systems.




 0
0