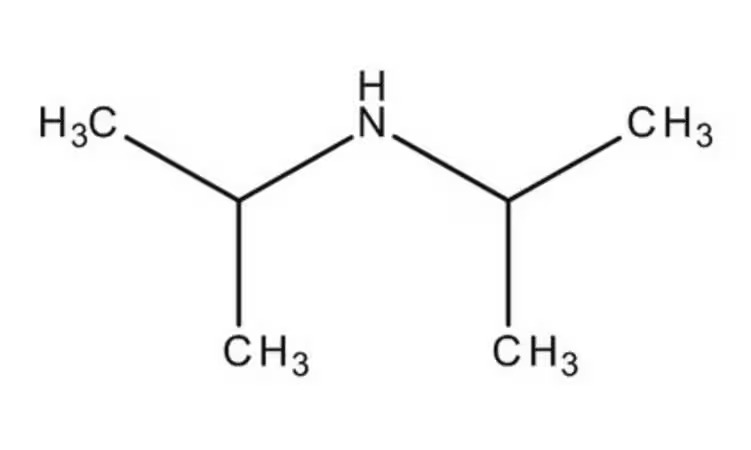
Specifications:
| Application | Organic Synthesis | ||
| Storage Temperature | Ambient | ||
| Product Type | Laboratory Chemical | Forms | Liquid |
| Product Brand | Thermo Fisher Scientific™, Alfa Aesar | ||
| Product Grade | Analytical grade | Formula | C₆H₁₅N |
Specifications:
| Feature | Test Results |
|---|---|
| Assay | ≥99% |
| Appearance | Clear liquid |
| Infrared Spectrum | Conforms |
| Refractive Index (20°C, 589 nm) | 1.3910 to 1.3930 |
| Water Content | ≤0.2% |
| GC Purity | ≥99.0% |
| Color Scale | ≤10 APHA |
Diisopropylamine (DIPA) is a high-purity aliphatic secondary amine widely used as a base in organic synthesis. It is 99+% pure, making it ideal for precise chemical reactions and industrial applications. DIPA is particularly known for its role as a reagent in the synthesis of furan derivatives, dihydrofuran, and butanolide derivatives. It also serves as an effective catalyst in reactions such as aryl disulfide formation via oxidative coupling and in Pd-catalyzed cross-coupling reactions like Heck and Sonogashira reactions.
In addition, diisopropylamine is used in the preparation of lithium diisopropylamide (LDA), a strong non-nucleophilic base frequently used in deprotonation reactions in organic chemistry.
Key Features:
-
High Purity:
- With ≥99% purity, diisopropylamine ensures consistent results in organic reactions and synthetic applications.
-
Versatile Reactant:
- Acts as a reagent in the synthesis of furan derivatives, aryl disulfides, and cross-coupling reactions.
-
Efficient Catalyst:
- Used as a catalyst in oxidative coupling reactions, facilitating the formation of aryl disulfides.
-
Non-Nucleophilic Base:
- Used in the preparation of lithium diisopropylamide (LDA), which is employed for deprotonation reactions in organic synthesis.
-
Flammable and Corrosive:
- As a flammable liquid (GHS02) and corrosive (GHS05), proper handling and storage precautions are required.
Applications:
-
Organic Synthesis:
- Diisopropylamine is used as a base for various organic reactions, including deprotonation and catalysis.
-
Synthesis of Furan Derivatives:
- Employed in the synthesis of furan, dihydrofuran, and butanolide derivatives through reactions with γ-ketothioesters.
-
Catalysis of Aryl Disulfides:
- Acts as a catalyst in the oxidative coupling of aryl thiols to prepare aryl disulfides.
-
Cross-Coupling Reactions:
- Used as a base in Pd-catalyzed reactions such as Heck and Sonogashira reactions, critical in carbon-carbon bond formation.
-
Preparation of Lithium Diisopropylamide (LDA):
- A precursor to LDA, which is used as a strong, non-nucleophilic base in organic reactions.
-
Pharmaceutical Synthesis:
- Plays a key role in the synthesis of pharmaceutical intermediates and in the production of medicinal compounds.
Packaging Options:
| Catalog Number | Pack Size | Packaging |
|---|---|---|
| A10280.AE | 100 mL | Glass bottle |
| A10280.0F | 2.5 L | Glass bottle |
Storage:
- Store at ambient temperature in a cool, dry place to maintain stability.
Thermo Fisher Scientific Diisopropylamine 99+% is an essential reagent for organic synthesis, especially in cross-coupling reactions, deprotonation processes, and the synthesis of valuable chemical intermediates. With its high purity, versatility, and non-nucleophilic base properties, it is a crucial tool in pharmaceutical research, polymer chemistry, and biochemical synthesis.
- Pack Size: 2.5L 100 mL




 0
0