Liofilchem® SensiTest Gram-Positive
Catalog No :
CAS Number :
Brand :
In Stock
System for the susceptibility testing of
Gram-positive bacteria
Specifications:
| Application | Microbial Identification/AST | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Microbial ID System | Forms | Kit with Various components |
| Product Brand | Liofilchem | ||
| Product Grade | Microbiology grade | ||
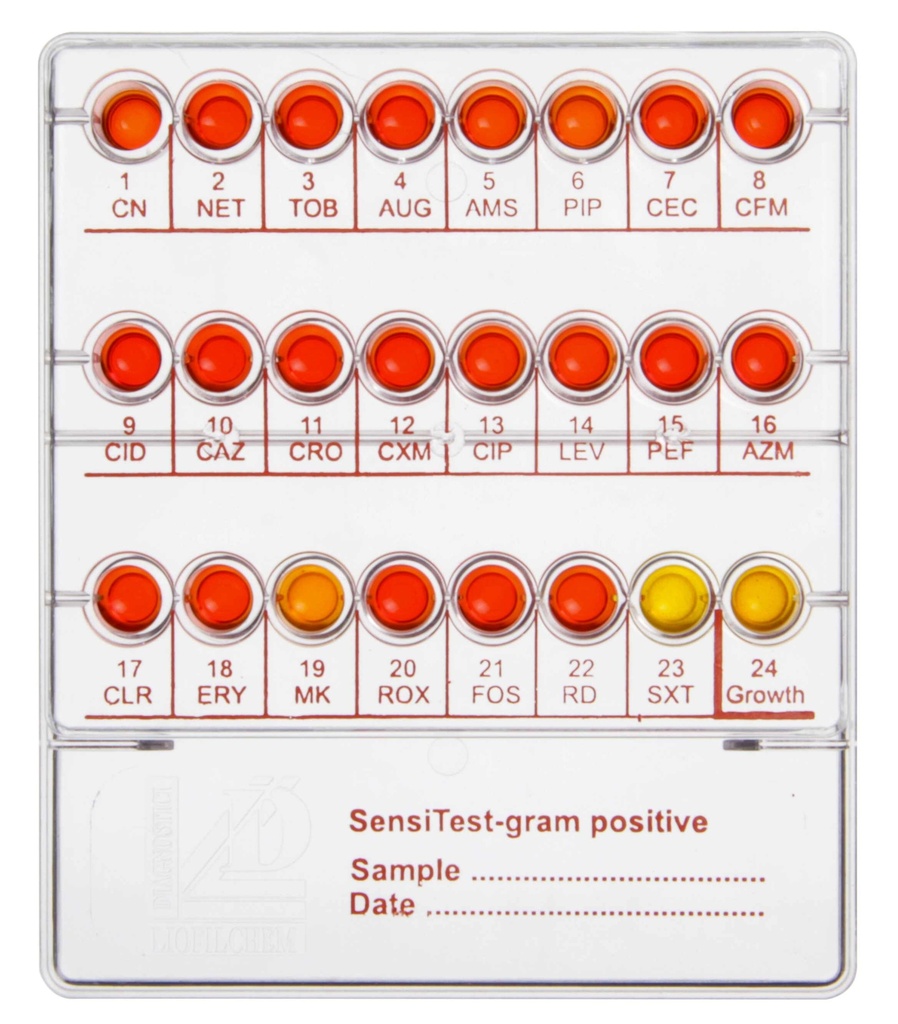
The Sensi Test Gram-Positive System is a diagnostic tool specifically designed for antimicrobial susceptibility testing of gram-positive bacteria, including Staphylococci, Streptococci, and gram-positive bacteria of veterinary origin. This system enables the determination of bacterial sensitivity to antibiotics using the broth microdilution method. It contains 24 wells, 23 of which are preloaded with desiccated antibiotics, and 1 growth control well. The system provides a qualitative assessment of bacterial resistance by evaluating the color change in the wells, classifying isolates as Sensitive (S), Intermediate (I), or Resistant (R).
Kit Components
Each kit contains:
- 20 Test Systems: Microplates with 24 pre-filled wells containing specific antibiotics and growth media.
- 20 Vials of Inoculum Broth (7 mL/vial): Used for bacterial suspension preparation.
- Instruction Manual: Detailed guidelines for handling, testing, and interpreting results.
- 20 Test Result Forms: Used to document and report susceptibility results.
Antibiotics in the System
The following antibiotics are included in the 24-well plate, representing various classes:
- Gentamicin (CN): 8 μg/mL
- Netilmicin (NET): 32 μg/mL
- Tobramycin (TOB): 8 μg/mL
- Amoxicillin/Clavulanic Acid (AUG): 8/4 μg/mL
- Ampicillin/Sulbactam (AMS): 32/16 μg/mL
- Piperacillin (PIP): 16 μg/mL
- Cefaclor (CEC): 32 μg/mL
- Cefixime (CFM): 32 μg/mL
- Cefonicid (CID): 32 μg/mL
- Ceftazidime (CAZ): 32 μg/mL
- Ceftriaxone (CRO): 64 μg/mL
- Cefuroxime (CXM): 32 μg/mL
- Ciprofloxacin (CIP): 4 μg/mL
- Levofloxacin (LEV): 8 μg/mL
- Pefloxacin (PEF): 8 μg/mL
- Azithromycin (AZM): 8 μg/mL
- Clarithromycin (CLR): 8 μg/mL
- Erythromycin (ERY): 8 μg/mL
- Miokamycin (MK): 8 μg/mL
- Roxithromycin (ROX): 8 μg/mL
- Fosfomycin (FOS): 200 μg/mL
- Rifampicin (RD): 4 μg/mL
- Co-trimoxazole (SXT): 8 μg/mL
- Growth Control Well (C): Contains only culture medium and growth indicator for viability assessment.
Principle of the Test
- Broth Microdilution Method: The test determines bacterial sensitivity by assessing growth or inhibition in wells containing specific antibiotics.
- Growth Indicator: Each well contains a growth indicator to signal bacterial viability.
- Red: No growth (Sensitive).
- Orange: Partial growth (Intermediate).
- Yellow: Significant growth (Resistant).
- Growth Control Well: Ensures bacterial viability and test accuracy.
Applications
This system is suitable for:
- Determining susceptibility of Staphylococcus and Streptococcus species.
- Testing gram-positive bacteria isolated from clinical and veterinary samples.
- Identifying resistance in bacterial isolates to inform targeted antimicrobial therapy.
Key Features and Benefits
- Comprehensive Testing: Covers a wide spectrum of antibiotics across multiple classes.
- Ease of Use: Ready-to-use microplates eliminate the need for manual antibiotic preparation.
- Reliability: Produces standardized results that align with EUCAST and CLSI guidelines.
- Growth Control Well: Ensures proper microbial viability for accurate interpretation.
- Compatible with Various Samples: Can be used with isolates from clinical, environmental, or veterinary sources.
- Time-Efficient: Results are available after 18–24 hours of incubation.
Preparation and Test Procedure
- Sample Preparation:
- Isolate bacteria from selective or non-selective media.
- Prepare a bacterial suspension equivalent to a 0.5 McFarland standard using Suspension Broth (20089) or Physiological Solution (20095).
- Inoculation:
- Add 0.2 mL of the bacterial suspension to the Inoculum Broth provided in the kit.
- Dispense 0.2 mL (4 drops) of the prepared inoculum into each well of the system.
- Incubation:
- Cover the plate with the lid provided.
- Incubate at 36°C ± 1°C for 18–24 hours.
- Result Interpretation:
- Observe the color change in each well and compare to Table 2.
- Transcribe results onto the ANTIBIOGRAMMA form provided.
- Interpret results based on EUCAST/CLSI breakpoints.
Storage and Stability
- Storage Conditions: Store at 2–8°C in the original packaging, away from light.
- Stability: Valid until the expiration date printed on the label when stored under recommended conditions.
- Transport Stability: Can tolerate temporary transport conditions of 18–25°C for 4 days or 35–39°C for 48 hours without compromising performance.
Quality Control
The system undergoes rigorous testing with reference strains to ensure batch-to-batch consistency:
- Staphylococcus aureus ATCC® 25923
- Streptococcus pneumoniae ATCC® 49619
Precautions
- For In Vitro Diagnostic Use only.
- Handle samples and used materials as potentially infectious.
- Only trained personnel should operate the system.
- Disinfect and dispose of materials per laboratory safety guidelines.
Packaging Information
- REF: 76020
- Kit Size: 20 tests
- Inoculum Broth: 20 vials, 7 mL each
- Storage Temperature: 2–8°C
The Sensi Test Gram-Positive System is a reliable, easy-to-use diagnostic tool for clinical microbiology laboratories to evaluate the susceptibility of gram-positive bacterial pathogens effectively and efficiently.
- Pack Size: for 20 Tests for 4 Tests




 0
0