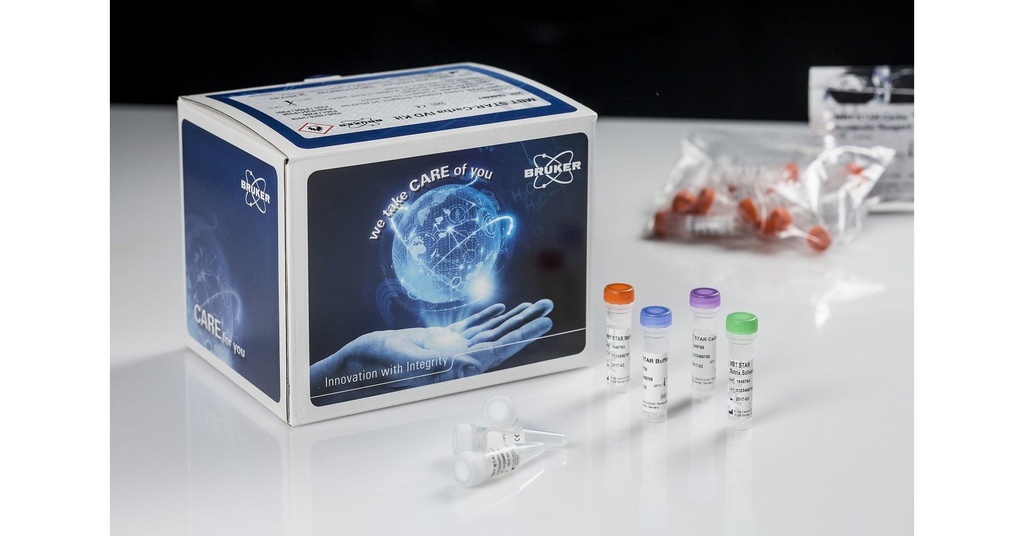
Specifications:
| Application | MALDI-MS | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Assay Kits | Forms | Liquid |
| Product Brand | Bruker | ||
| Product Grade | Microbiology grade | ||
The Bruker MALDI Biotyper® MBT Mycobacteria RUO Kit offers an innovative approach to identifying Mycobacterium spp., including the Mycobacterium tuberculosis complex (MTC) and nontuberculous mycobacteria (NTM). By combining a robust reference spectrum library with advanced software, it provides a precise, streamlined, and biosafe workflow for mycobacteria identification.

Key Features
- Comprehensive Mycobacteria Library:
- Covers 182 mycobacteria species out of the 201 currently known.
- Includes spectra for pathogenic and opportunistic mycobacteria species.
- Optimized Workflow:
- Designed for use with both solid and liquid cultures.
- Eliminates the need for boiling, utilizing a room-temperature inactivation process for enhanced safety.
- Advanced Software Module:
- Handles the complex spectra associated with mycobacteria.
- Automatically compares spectra to the dedicated MBT Mycobacteria Library for species-level identification.
- Biosafety Compliance:
- Utilizes an Inactivation Reagent to ensure safe handling of biosafety level 3 organisms.
- Includes a mechanical disruption step for consistent sample preparation.
- Fast and Accurate:
- Reduces the lengthy turnaround times of traditional methods.
- Provides a cost-effective alternative to gene sequencing without sacrificing accuracy.
Applications
- Clinical Microbiology:
- Differentiates between MTC and NTM for targeted antimicrobial therapy.
- Facilitates the diagnosis of mycobacterial infections, including drug-resistant tuberculosis.
- Antimicrobial Resistance Monitoring:
- Supports identification of mycobacteria with different susceptibility profiles, aiding in the selection of effective treatments.
- Public Health and Research:
- Tracks emerging trends in nontuberculous mycobacteria infections.
- Monitors the spread of antibiotic-resistant strains of mycobacteria.
- Hospital Laboratories:
- Enhances the speed and accuracy of mycobacteria identification, improving infection control measures.
- Veterinary and Environmental Applications:
- Identifies mycobacteria in environmental samples or veterinary diagnostics.
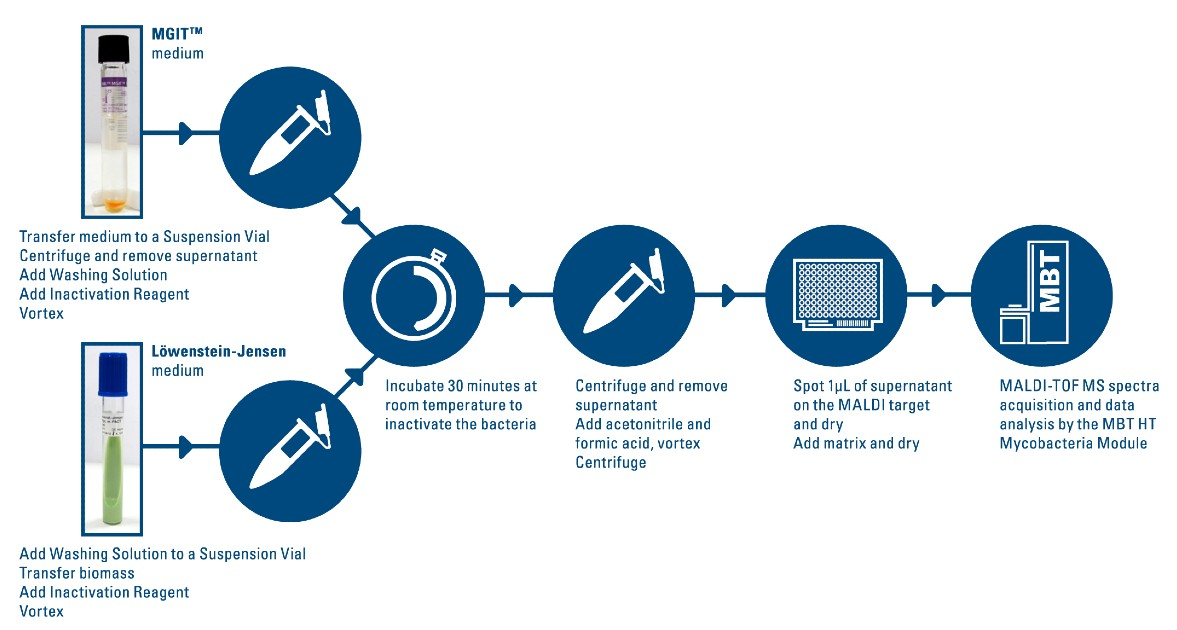
Workflow
- Sample Preparation:
- Cultivate samples in liquid or solid media.
- Treat samples with the Inactivation Reagent at room temperature.
- Mechanically disrupt cell aggregates for uniform processing.
- Mass Spectrometry Analysis:
- Acquire spectra using the MALDI Biotyper®.
- Compare data against the MBT Mycobacteria Library.
- Data Interpretation:
- The MBT HT Mycobacteria Module analyzes and identifies mycobacteria species.
- Results are accurate, sensitive, and ready for reporting.
Benefits
- Improved Diagnostic Accuracy: Identifies 182 species of mycobacteria with high sensitivity and specificity.
- Enhanced Biosafety: Inactivation process ensures safe handling of high-risk organisms.
- Time Efficiency: Drastically reduces identification time compared to culture-based methods.
- Cost-Effectiveness: Provides an affordable alternative to sequencing technologies.
- Ease of Use: Integrates with the MALDI Biotyper® platform for seamless microbiological workflows.
Specific Use Cases
- MTC vs. NTM Differentiation:
- Rapidly identifies tuberculosis-causing species versus nontuberculous opportunistic pathogens.
- Example: Differentiating Mycobacterium tuberculosis from Mycobacterium avium in pulmonary infections.
- NTM Outbreak Management:
- Identifies species responsible for hospital-acquired NTM infections, such as Mycobacterium abscessus.
- Aids in implementing infection control measures.
- Research on Emerging Mycobacteria:
- Monitors rare or novel mycobacteria species in environmental or clinical settings.
Product Options
- MBT HT Mycobacteria IVD Module:
- Designed for professional diagnostic use (IVD-CE certified).
- Includes software module and mycobacteria reference library.
- MBT HT Mycobacteria Module (RUO):
- For research use only, not intended for clinical diagnostics.
- Offers the same robust library and software for non-diagnostic applications.
The MBT Mycobacteria IVD Kit redefines mycobacteria identification by addressing the challenges of speed, accuracy, and biosafety. Its advanced tools empower microbiologists to differentiate mycobacteria species efficiently, supporting better patient outcomes and effective infection control strategies.




 0
0