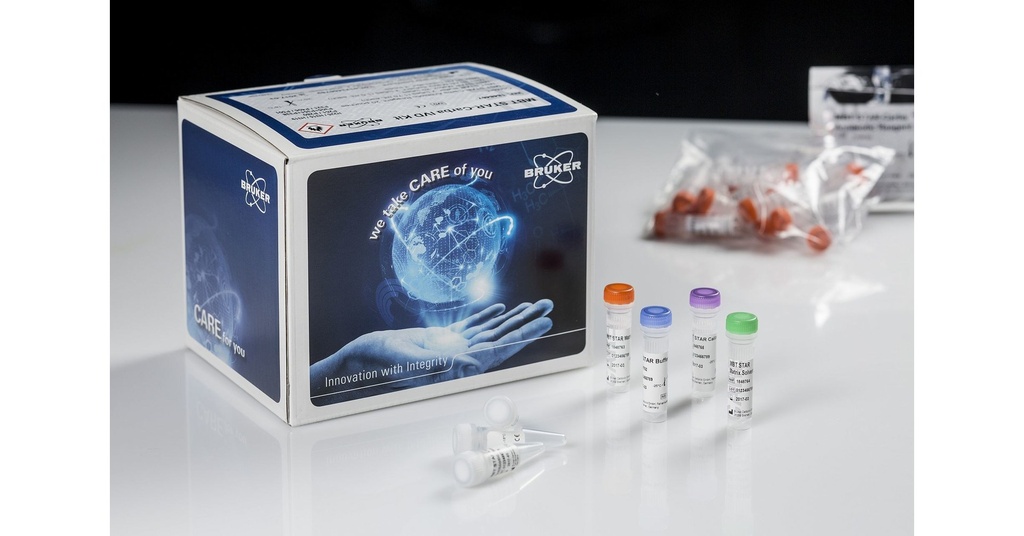
Specifications:
| Application | MALDI-MS |
| Storage Temperature | 2-8°C |
| Product Type | Assay Kits |
| Product Brand | Bruker |
| Product Grade | Microbiology grade |
The Bruker MALDI Biotyper® MBT STAR®-Cepha IVD Kit is designed to combat resistance to third-generation cephalosporins by identifying the enzymatic hydrolysis of the beta-lactam ring in cephalosporin antibiotics. It supports the detection of extended-spectrum beta-lactamases (ESBL) and AmpC beta-lactamases, offering critical diagnostic insights into multidrug-resistant (MDR) Gram-negative bacteria.
Key Features
- Rapid Resistance Detection:
- Swiftly identifies cephalosporin resistance directly from positive blood cultures or isolated colonies.
- Results are available within 30-60 minutes, enabling prompt decision-making.
- Comprehensive Beta-Lactamase Detection:
- ESBL: Detects plasmid-mediated resistance enzymes such as TEM, SHV, and CTX-M.
- AmpC: Identifies chromosomal and plasmid-mediated resistance genes, including AmpC, FOX, LAT, DHA, and CMY.
- Automated Analysis:
- The MBT HT STAR®-BL module simplifies data interpretation with color-coded indicators and clear visualization of results.
- Flexible Workflow:
- Compatible with both positive blood culture pellets and plate cultures.
- Integrates seamlessly with the MALDI Biotyper® platform for a unified diagnostic solution.
- IVD-CE Certified:
- Approved for in vitro diagnostic use in CE regions, supporting compliance with stringent healthcare standards.
Applications
- Cephalosporin Resistance Monitoring:
- Identifies resistance mechanisms in clinical isolates, guiding therapy for infections caused by resistant pathogens.
- Hospital Infection Control:
- Detects resistant Gram-negative Enterobacterales to prevent the spread of MDR organisms in healthcare settings.
- Sepsis Diagnostics:
- Provides rapid resistance profiling after a positive blood culture alert, improving antimicrobial stewardship.
- Multidrug-Resistant Pathogen Surveillance:
- Tracks the emergence and prevalence of ESBL- and AmpC-producing bacteria in clinical and research settings.
Workflow
- Sample Preparation:
- A positive blood culture or isolated bacterial colony is incubated with a beta-lactam antibiotic (e.g., third-generation cephalosporins).
- Mass Spectrometry Analysis:
- After incubation, the sample is analyzed using the MALDI Biotyper®, which measures the mass shift caused by beta-lactam ring hydrolysis.
- Data Interpretation:
- The MBT HT STAR®-BL software module computes the ratio of hydrolyzed to intact antibiotic molecules, presenting results in an easy-to-read, color-coded format.
- Report Generation:
- Users receive a comprehensive report detailing enzyme activity status and resistance profiles.
Use Cases
- Detection of ESBL Resistance:
- Example: Identifying Escherichia coli or Klebsiella pneumoniae isolates with CTX-M-mediated resistance in bloodstream infections.
- AmpC Beta-Lactamase Surveillance:
- Example: Monitoring chromosomal AmpC resistance in Enterobacter cloacae during an outbreak.
- Therapeutic Guidance:
- Supports clinicians in selecting effective antibiotics, minimizing treatment failures, and preventing overuse of broad-spectrum drugs.
Benefits
- Speed: Results are available much faster than conventional phenotypic methods.
- Accuracy: Provides high confidence in detecting specific resistance mechanisms.
- Ease of Use: Automated software simplifies analysis and reporting.
- Cost-Effectiveness: Combines identification and resistance testing on a single MALDI-TOF platform.
- Enhanced Stewardship: Improves patient outcomes by promoting targeted therapy.
By enabling rapid detection of third-generation cephalosporin resistance, the MBT STAR®-Cepha IVD Kit empowers microbiology labs to improve diagnostic efficiency and combat the growing threat of antibiotic resistance.




 0
0