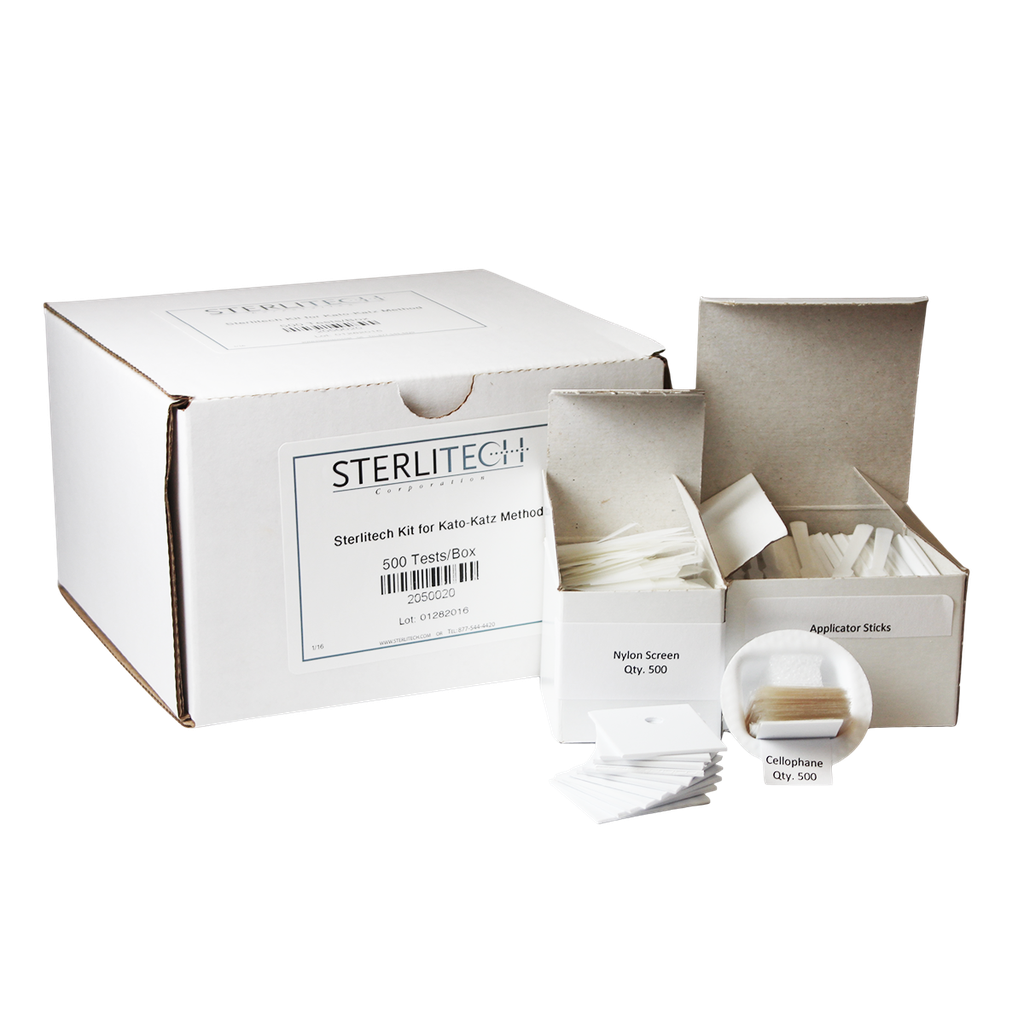
Sterlitech Kit For Kato-Katz Method, 500 Tests/Box
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Storage Temperature | Room Temperature |
| Product Type | Test Kit |
| Product Brand | Sterlitech |
| Product Grade | Medical grade |
The Sterlitech Kato-Katz Method Kit is designed for efficient and standardized diagnosis of intestinal schistosomiasis and soil-transmitted helminthiasis (STH) using fecal smear microscopy. The kit is built to adhere to World Health Organization (WHO) guidelines and contains materials sufficient for 500 tests.
Kit Contents
Each kit includes 500 pieces each of:
- Applicator Sticks:
- Used for transferring and leveling fecal samples.
- Nylon Screens (100 mesh):
- For sieving fecal material to ensure uniform sample preparation.
- Templates (Calibrated to 41.7 mg):
- Ensures precise and consistent sample size for accurate analysis.
- Hydrophilic Cellophane Strips:
- Soaked in glycerol-dye solution to act as coverslips for prepared slides.
Additional Items Required (Not Included in Kit)
- Microscope Slides (500 pieces):
- Used for mounting the prepared fecal smears.
- Flat-Bottomed Sample Jars:
- To hold fecal samples during preparation.
- Forceps:
- For handling materials like cellophane strips.
- Absorbent Tissue Paper:
- To remove excess glycerol from slides.
- Newspaper or Similar Material:
- Serves as a working surface during sample preparation.
- Glycerol-Dye Solution:
- 1 mL of 3% Malachite Green or Methylene Blue mixed in 200 mL of 50% or greater glycerol solution.
Applications
The Kato-Katz Method is primarily used for:
- Diagnosis of Intestinal Parasites:
- Detects eggs of intestinal helminths such as Ascaris lumbricoides, Trichuris trichiura, and Schistosoma species.
- Epidemiological Studies:
- Determines the prevalence and intensity of infections in a population.
- Monitoring and Evaluation:
- Measures the impact of treatment programs for controlling helminth infections.
Instructions for Use
- Preparation of Cellophane Strips:
- Soak strips in glycerol-dye solution for at least 24 hours before use.
- Sample Collection:
- Place a small fecal sample on scrap paper.
- Sieving:
- Press a nylon screen on top of the fecal sample and scrape across the screen using the flat side of the applicator stick to obtain sieved material.
- Template Application:
- Place the template on a clean microscope slide and fill the hole with the sieved fecal material. Level it with the applicator stick.
- Template Removal:
- Remove the template carefully, ensuring all fecal material remains on the slide.
- Cellophane Cover:
- Cover the sample with a glycerol-soaked cellophane strip and remove excess glycerol with absorbent tissue if necessary.
- Slide Preparation:
- Invert the slide and press it against a smooth surface to evenly spread the fecal sample.
- Gently slide the microscope slide sideways to prevent cellophane separation.
- Incubation:
- Allow the slide to incubate at room temperature for 24 hours before examination.
Reading the Slides
- Hookworm Eggs:
- Examine slides within 30–60 minutes of preparation to prevent degradation.
- Other Helminth Eggs:
- Read slides after 24 hours of incubation for Ascaris, Trichuris, and Schistosoma eggs.
Advantages
- Standardized Workflow:
- Ensures consistent sample sizes and preparation, leading to reliable results.
- Field-Friendly:
- Lightweight and portable, ideal for field-based studies and epidemiological surveys.
- Cost-Effective:
- Contains all essential materials for 500 tests, reducing additional costs.
- WHO Compliance:
- Follows WHO recommendations, ensuring global consistency in helminth diagnostics.
Kit Specifications
| Feature | Details |
|---|---|
| Capacity | 500 tests per box |
| Sample Size | 41.7 mg per test (WHO standard) |
| Mesh Size | 100 mesh (nylon screens) |
| Storage Requirements | Room temperature |
| Preparation Time | 24-hour pre-soak for cellophane strips |
Applications in Research and Healthcare
- Parasitology Studies: Monitoring helminth prevalence and intensity in populations.
- Public Health Programs: Assessing the success of deworming and other control measures.
- Clinical Diagnostics: Identifying helminth infections for targeted treatment.
References
- World Health Organization (WHO), "Basic Laboratory Methods in Parasitology" (1991).
- "Action Against Worms," WHO, 2008.
This kit is an essential diagnostic tool for laboratories, researchers, and field teams involved in parasitology and public health initiatives. It ensures accurate, reliable, and standardized testing for intestinal helminths across diverse settings.




 0
0