Sigma-AldrichSorbitol United States Pharmacopeia (USP) Reference Standard, 125mg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | chromatography | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Calibration Standard | Forms | Solid |
| Product Brand | Sigma-Aldrich | ||
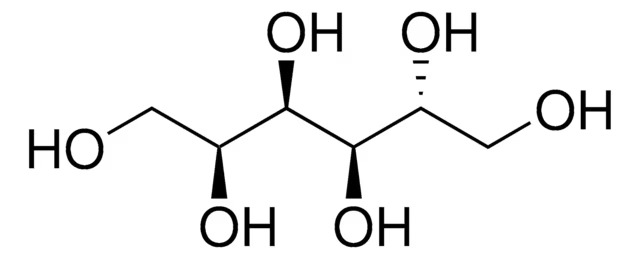
| Product Grade | HPLC Grade | Formula | HOH₂C(CH(OH))₄CH₂OH |
Sigma-Aldrich Sorbitol USP Reference Standard (125 mg) is a pharmaceutical primary standard used for quality control and analytical testing in pharmaceutical applications. It is specified by the United States Pharmacopeia (USP) and is suitable for use in compendial tests and assays.
This neat format sorbitol standard is commonly used to prepare standard and system suitability solutions for assay validation using liquid chromatography (LC) with refractive index (RI) and UV detection. It is widely applied in pharmaceutical formulations, excipient analysis, and impurity testing.
Key Features & Benefits
1. High-Purity USP Reference Standard
- Meets USP compendial requirements for pharmaceutical applications.
- Provides high accuracy and consistency in analytical testing.
- Pharmaceutical primary standard grade, ensuring reliable assay results.
2. Optimized for Liquid Chromatography (LC) Analysis
- Suitable for system suitability solutions and assay preparation.
- Compatible with LC with refractive index (RI) detection for sorbitol analysis.
- Can be used for polyol impurity testing in xylitol via LC with UV detection at 192 nm.
3. Versatile Use in Pharmaceutical Quality Control
- Supports the analysis of sorbitol-containing formulations.
- Used in the evaluation of noncrystallizing sorbitol solutions.
- Helps ensure compliance with USP monographs and regulatory guidelines.
Specifications
| Property | Details |
|---|---|
| Grade | Pharmaceutical Primary Standard |
| Chemical Name | Sorbitol (C₆H₁₄O₆) |
| Molecular Formula | C₆H₁₄O₆ |
| Format | Neat |
| Purity | USP Reference Standard |
| Melting Point | 98-100°C (lit.) |
| Vapor Density | <1 (vs air) |
| Vapor Pressure | <0.1 mmHg (25°C) |
| Solubility | Highly soluble in water |
| Storage Conditions | Room temperature |
| Application | Pharmaceutical testing, assay validation, impurity testing |
Applications
1. Pharmaceutical Analysis & Quality Control
- Used as a standard in sorbitol-related USP monographs:
- Sorbitol Solution
- Sorbitol
- Noncrystallizing Sorbitol Solution
- Sorbitol Sorbitan Solution
- Ensures batch-to-batch consistency in pharmaceutical formulations.
2. Liquid Chromatography (LC) Analysis
- Helps in assay validation and system suitability tests.
- Used in LC-RI detection for sorbitol quantification.
- Detects polyol impurities in xylitol using LC-UV at 192 nm.
3. Excipients & Impurity Testing
- Used in analysis of excipient quality and purity.
- Ensures regulatory compliance for sorbitol-containing formulations.
- Supports research and development of pharmaceutical products.
Storage & Handling
- Store at room temperature.
- Use only for analytical testing—not for human or animal administration.
- Follow USP guidelines for preparation and usage.
Summary
The Sigma-Aldrich Sorbitol USP Reference Standard (125 mg) is a high-purity pharmaceutical primary standard used for compendial tests, LC assay validation, and impurity detection. It provides reliable and reproducible results in pharmaceutical quality control applications, ensuring compliance with USP monographs.




 0
0