Thermo Scientific™ Oxoid™ Vancomycin VA Antimicrobial Susceptibility discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Thermo Fisher Scientific™, Oxoid |
| Product Grade | Microbiology grade |
Thermo Scientific™ Oxoid™ Vancomycin Antimicrobial Susceptibility Discs are high-quality diagnostic tools used for manual antimicrobial susceptibility testing (AST) via the disc diffusion method. Designed for the semi-quantitative evaluation of bacterial resistance or susceptibility to vancomycin, these discs are widely used in clinical microbiology for monitoring resistance in Gram-positive bacteria, particularly Staphylococcus aureus, Enterococcus spp., and Streptococcus spp.
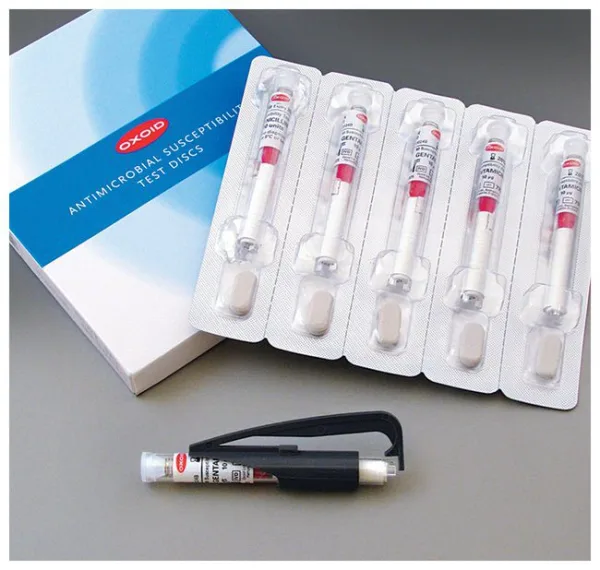
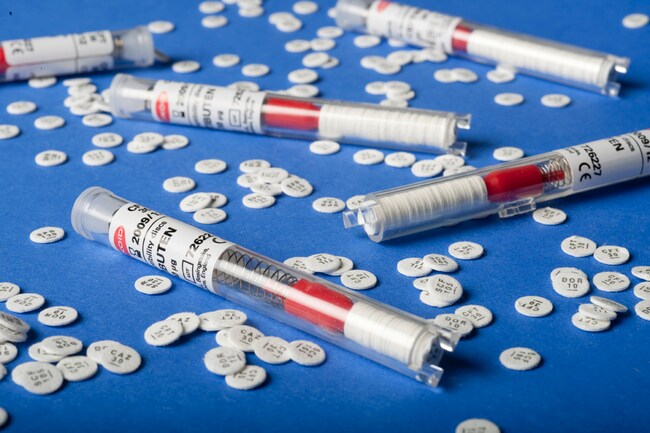
Available in 5 µg (CT0188B) and 30 µg (CT0058B) concentrations, each disc is housed in a spring-loaded, shatterproof cartridge to ensure easy handling and consistent delivery. The cartridges are individually sealed with desiccant, maintaining a moisture level below 2% to ensure long-term stability and disc integrity.
These discs comply with BSAC guidelines, offering accurate zone-of-inhibition readings that support classification of test organisms as susceptible, intermediate, or resistant. In addition to quantitative data, the vancomycin discs also offer visual insights into potential inoculum issues, contamination, resistant subpopulations, and antagonistic or synergistic effects when tested in combination with other antimicrobial agents.
Key Features and Benefits
- Dual Strength Availability: Supplied in 5 µg and 30 µg concentrations to support varied testing needs.
- Pharmacopoeia Compliance: Conforms to BSAC standards for dependable and reproducible test outcomes.
- Precision Cartridge System: Spring-loaded, shatterproof cartridges with end-of-cartridge indicators enhance usability.
- Stabilized Packaging: Each cartridge is individually sealed with desiccant to preserve potency and shelf life.
- Visual Interpretation: Enables identification of contamination, inoculum density, and resistance mechanisms.
- Broad Utility: Suitable for use in diagnostic laboratories, surveillance programs, pharmaceutical QC, and hospital AST workflows.
Product Specifications
| Attribute | Details |
|---|---|
| Product Name | Oxoid™ Vancomycin Antimicrobial Susceptibility Discs |
| Antibiotic Code | VA |
| Antimicrobial Agent | Vancomycin |
| Available Concentrations | 5 µg (CT0188B), 30 µg (CT0058B) |
| Disc Quantity | 50 discs per cartridge; 5 cartridges per pack |
| Compliance | BSAC guidelines |
| Test Type | Antimicrobial Susceptibility Testing (Disc Diffusion) |
| Packaging Format | Spring-loaded cartridge with desiccant seal |
| Intended Use | In Vitro Diagnostic Use |
| Storage | As per manufacturer’s instructions to maintain stability |
| Unit Size | Each |
Intended Use
These discs are intended for in vitro semi-quantitative AST by agar diffusion to determine bacterial susceptibility to vancomycin. They aid in the clinical decision-making process by guiding appropriate antimicrobial therapy.
Thermo Scientific™ Oxoid™ Vancomycin Antimicrobial Susceptibility Discs provide a standardized, compliant, and highly reliable method for phenotypic antimicrobial susceptibility testing. Their superior cartridge design, consistent potency, and validated pharmacopoeial compliance make them a trusted tool for laboratories engaged in the detection and monitoring of vancomycin resistance among Gram-positive pathogens. Ideal for routine diagnostics, hospital surveillance, and pharmaceutical testing, these discs ensure accurate and reproducible results with every application.
- Drug Concentration: 5 µg 30 µg




 0
0