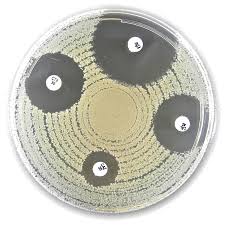
Liofilchem® Trimethoprim TM, Antimicrobial Susceptibility Discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Trimethoprim (TM) Antimicrobial Susceptibility Discs, available in 2.5 µg and 5 µg potencies, are high-quality, sterile paper discs designed for in vitro antimicrobial susceptibility testing (AST) using the Kirby-Bauer disk diffusion method. These discs are impregnated with a precise amount of trimethoprim, a folic acid synthesis inhibitor that blocks bacterial dihydrofolate reductase, impairing DNA replication and cell survival.
Trimethoprim is active primarily against Gram-negative and some Gram-positive bacteria, and these discs are commonly used in both clinical diagnostics and antimicrobial resistance research. Manufactured under ISO 9001 and ISO 13485 quality systems, Liofilchem® discs ensure consistent potency and reproducibility in zone-of-inhibition measurements.
Key Features
-
Available dosages:
- 2.5 µg disc: Suitable for high-sensitivity detection or research applications.
- 5 µg disc: Standard dosage for routine clinical AST workflows.
- Broad-spectrum activity: Active against Escherichia coli, Proteus spp., Shigella spp., Salmonella spp., Staphylococcus aureus, and Haemophilus influenzae.
- Mechanism-based testing: Specifically inhibits dihydrofolate reductase, making it useful for studying folate-pathway-targeted antibiotics.
- Used alone or for TMP-SMX comparison: Enables individual susceptibility assessment apart from the commonly combined trimethoprim-sulfamethoxazole formulation.
- CLSI/EUCAST compatible: Results are interpretable using established zone diameter guidelines.
- Sterile and shelf-stable: Packaged in sealed cartridges or canisters with desiccant to maintain potency.
- Color-coded labeling: Facilitates quick identification in high-throughput laboratories.
Applications and Use Cases
1. Clinical Microbiology
- Susceptibility testing of urinary, enteric, and respiratory pathogens, especially E. coli and Staphylococcus aureus.
- Comparative testing with TMP-SMX to determine resistance to the trimethoprim component alone.
2. Infection Control and AMR Surveillance
- Detect and track resistance trends in sulfonamide and trimethoprim pathways.
- Support outbreak investigations involving Shigella or Salmonella.
3. Veterinary and Environmental Microbiology
- AST in zoonotic and commensal bacteria from livestock and water sources.
- Monitoring environmental reservoirs for trimethoprim resistance genes.
4. Research and Pharmaceutical Development
- Use in gene resistance studies (e.g., dfrA, dfrB genes).
- Evaluate new antibiotic combinations or test novel dihydrofolate reductase inhibitors.
Use Instructions
- Medium: Mueller-Hinton Agar (MHA) is recommended.
- Inoculum Preparation: Prepare a bacterial suspension equivalent to 0.5 McFarland standard.
- Inoculation: Swab the agar plate evenly with the inoculum.
- Disc Placement: Place one 2.5 µg or 5 µg Trimethoprim disc on the agar using sterile forceps or a dispenser.
-
Incubation:
- Temperature: 35 ± 2 °C
- Time: 16–18 hours under aerobic conditions
-
Interpretation:
- Measure the diameter of the inhibition zone in millimeters.
- Compare to CLSI or EUCAST breakpoints, or validated in-house criteria.
Technical Specifications
| Specification | Detail |
|---|---|
| Antibiotic | Trimethoprim (TM) |
| Disc Potency | 2.5 µg or 5 µg |
| Disc Diameter | 6 mm |
| Disc Format | Sterile filter paper disc |
| Packaging | 50 discs (cartridge) / 250 discs (canister) |
| Storage Conditions | 2–8 °C (refrigerated, sealed, dry) |
| Shelf Life | 24–36 months from date of manufacture |
Quality Control
Tested using standard ATCC strains under CLSI/EUCAST conditions:
| Control Organism | Expected Zone Diameter |
|---|---|
| Escherichia coli ATCC® 25922 | Within CLSI range |
| Staphylococcus aureus ATCC® 25923 | CLSI-compliant result |
| Haemophilus influenzae ATCC® 49247 | Susceptibility verified |
Liofilchem® Trimethoprim TM Discs (2.5 µg, 5 µg) are essential tools for assessing bacterial susceptibility to trimethoprim, supporting precise antimicrobial therapy decisions, resistance monitoring, and comparative research. With ISO-certified quality, clear zone interpretability, and flexible dosing formats, these discs are well-suited for use in clinical, veterinary, and environmental microbiology labs.
- Drug Concentration: 5 µg 2.5 µg




 0
0