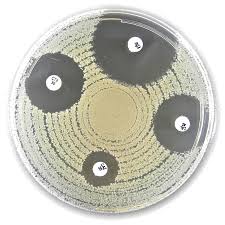
Liofilchem® Polymyxin B PB, Antimicrobial Susceptibility Discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Polymyxin B (PB) Antimicrobial Susceptibility Discs, available in 100 IU and 300 IU potencies, are high-quality paper discs used for in vitro antimicrobial susceptibility testing (AST) of Gram-negative bacteria. These discs are specifically designed for use with the Kirby-Bauer disk diffusion method, particularly in the detection of polymyxin B resistance among clinically significant multidrug-resistant Gram-negative bacilli, including Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae such as Klebsiella pneumoniae.
Manufactured under ISO 9001 and ISO 13485 certified processes, these discs provide consistent and reproducible zone diameters, making them ideal for clinical diagnostics, resistance surveillance, and research applications.
Key Features
-
Two dosage strengths:
- 100 IU: Commonly used for sensitive detection of polymyxin B activity in research and veterinary applications.
- 300 IU: Recommended for routine susceptibility testing in clinical microbiology for accurate resistance detection.
- Broad Gram-negative activity: Effective against non-fermenters and Enterobacteriaceae, excluding Gram-positive and anaerobic organisms.
- Critical for multidrug resistance (MDR) detection: Supports detection of last-resort resistance mechanisms in critical care settings.
- CLSI/EUCAST compliant: Designed for use with standardized disc diffusion testing methods.
- Sterile and shelf-stable: Packaged with desiccant in sealed cartridges or canisters to maintain potency.
- Color-coded labels: Allow easy identification and handling in high-throughput laboratories.
Applications and Use Cases
1. Clinical Microbiology
- Assessment of polymyxin B susceptibility in carbapenem-resistant Enterobacteriaceae (CRE) and extensively drug-resistant (XDR) pathogens.
- Routine susceptibility testing in critical infections caused by P. aeruginosa, K. pneumoniae, and A. baumannii.
2. Public Health and AMR Surveillance
- Screening for colistin/polymyxin resistance genes (e.g., mcr-1) in hospital-acquired and environmental isolates.
3. Veterinary Microbiology
- Testing of Gram-negative pathogens in veterinary clinical isolates, especially in poultry and swine diseases.
4. Pharmaceutical Research
- Evaluation of resistance mechanisms to polymyxin-class antibiotics.
- Support for developing novel polymyxin formulations and combination therapies.
Use Instructions
- Media: Use Mueller-Hinton Agar (MHA) as the standard base medium.
- Inoculum Preparation: Adjust bacterial suspension to a 0.5 McFarland turbidity standard.
- Inoculation: Evenly streak the agar plate using a sterile swab.
- Disc Application: Apply the 100 IU or 300 IU Polymyxin B disc using sterile forceps or a mechanical disc dispenser.
-
Incubation:
- Temperature: 35 ± 2 °C
- Duration: 16–18 hours in ambient air
-
Interpretation:
- Measure the inhibition zone diameter in millimeters.
- Compare results with CLSI or EUCAST interpretive criteria, where available.
Note: Because of limitations in diffusion, polymyxin B disk diffusion testing may be less reliable for certain isolates—broth microdilution remains the gold standard for polymyxin MIC determination.
Technical Specifications
| Specification | Detail |
|---|---|
| Antibiotic | Polymyxin B (PB) |
| Disc Potency | 100 IU or 300 IU |
| Disc Diameter | 6 mm |
| Format | Sterile filter paper disc |
| Packaging | 50 discs (cartridge) or 250 discs (canister) |
| Storage Conditions | 2–8 °C, dry, sealed with desiccant |
| Shelf Life | 24–36 months from manufacture |
Quality Control
Validated using internationally recognized control strains:
| Organism | Expected Inhibition Zone (mm) |
|---|---|
| Escherichia coli ATCC® 25922 | Within reference range |
| Pseudomonas aeruginosa ATCC® 27853 | Within reference range |
| Klebsiella pneumoniae (clinical) | For resistant strain screening |
Liofilchem® Polymyxin B PB Discs (100 IU, 300 IU) offer a reliable and standardized approach to polymyxin susceptibility testing, especially for multidrug-resistant Gram-negative infections. Whether used in routine hospital labs or for advanced AMR research, these discs provide the precision and performance necessary for guiding therapeutic decisions and monitoring resistance trends.
- Drug Concentration: 100 IU 300 IU




 0
0