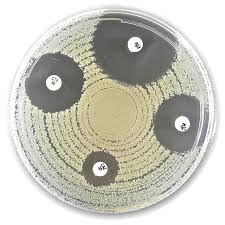
Liofilchem® Nitrofurantoin F, Antimicrobial Susceptibility Discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Liofilchem® Nitrofurantoin F Antimicrobial Suscsceptibility Discs are precision-manufactured filter paper discs impregnated with 100 µg or 300 µg of Nitrofurantoin, a broad-spectrum urinary tract antibiotic. These discs are used for the qualitative determination of antimicrobial susceptibility of bacterial isolates through the Kirby-Bauer disk diffusion method, as outlined in CLSI and EUCAST standards.
Nitrofurantoin is particularly effective against Escherichia coli, Enterococci, and other urinary tract pathogens. The 300 µg disc concentration is specifically useful for detecting low-level resistance mechanisms or in epidemiological surveillance, while the 100 µg disc is suitable for standard clinical susceptibility testing.
These discs are part of Liofilchem’s extensive IVD-certified range, ensuring batch-to-batch consistency, strict potency control, and international guideline compliance.
Key Features & Benefits
- ✅ Available in Two Potencies – 100 µg for routine susceptibility testing, 300 µg for enhanced resistance profiling
- ✅ CLSI & EUCAST Compliant – Developed and validated according to international standards
- ✅ IVD Certified – Manufactured under ISO 9001 and ISO 13485 quality systems
- ✅ Easy Identification – Discs are clearly labelled with concentration and antibiotic code
- ✅ Reliable & Reproducible – Accurate zone diameters with minimal lot-to-lot variability
- ✅ Long Shelf Life – Extended stability when stored properly at 2–8°C
- ✅ Validated Against Key Pathogens – Optimized for E. coli, Klebsiella spp., Enterococcus spp., and more
Applications
- Antimicrobial susceptibility testing in clinical microbiology laboratories
- Detection of Nitrofurantoin-resistant urinary tract pathogens
- Routine hospital antibiogram surveillance
- Research on emerging resistance in enteric bacteria
- Epidemiological studies and antimicrobial stewardship programs
Technical Specifications
| Parameter | Details |
|---|---|
| Active Ingredient | Nitrofurantoin F |
| Disc Concentrations | 100 µg, 300 µg |
| Format | Filter paper discs, 6 mm diameter |
| Number of Discs | Typically 50 discs/vial |
| Application Method | Kirby-Bauer disk diffusion |
| Compliance | CLSI, EUCAST |
| Classification | IVD – In Vitro Diagnostic Use |
| Organisms Tested | E. coli, Enterococcus spp., Klebsiella spp. |
| Quality Assurance | ISO 9001, ISO 13485 certified production |
| Storage Conditions | 2–8°C (Refrigerated) |
| Shelf Life | Typically 2–3 years from manufacture |
| Labeling | Color-coded, with clear antibiotic marking |
Zone Diameter Interpretive Standards
(Always refer to the most recent CLSI or EUCAST guidelines for interpretive criteria.)
| Pathogen | Disc | Interpretation |
|---|---|---|
| E. coli | ≥ 17 mm | Susceptible |
| Enterococcus faecalis | ≥ 20 mm | Susceptible |
| Klebsiella spp. | Varies | Usually Resistant |
The Liofilchem® Nitrofurantoin F Antimicrobial Susceptibility Discs provide a robust, validated solution for monitoring the efficacy of nitrofurantoin against key urinary tract pathogens. With availability in both 100 µg and 300 µg concentrations, these discs serve dual purposes: routine diagnostics and advanced resistance surveillance. Their IVD certification, consistent performance, and compliance with CLSI and EUCAST standards make them an essential tool for clinical microbiology and antimicrobial stewardship.
- Drug Concentration: 50 µg 300 µg 100 µg




 0
0