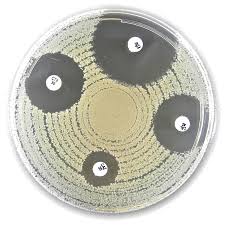
Liofilchem® Linezolid LNZ Antimicrobial Susceptibility Discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Reliable In Vitro Disc Diffusion Tools for Monitoring Linezolid Susceptibility in Gram-Positive Pathogens
Liofilchem® Linezolid (LNZ) Antimicrobial Susceptibility Discs are standardized, high-quality paper discs impregnated with 10 µg or 30 µg of linezolid, an oxazolidinone-class antibiotic used for the treatment of Gram-positive bacterial infections, including those caused by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multi-drug resistant Streptococcus pneumoniae.
These discs are designed for use in the Kirby-Bauer disc diffusion method on Mueller-Hinton Agar, providing accurate and reproducible results in compliance with CLSI M100 and EUCAST interpretive guidelines. Manufactured under ISO 9001 and ISO 13485 certifications, they are essential for clinical microbiology labs, hospital infection control units, and research laboratories monitoring resistance trends.
Key Features & Benefits
-
Available in Two Concentrations:
- LNZ 10 µg – For specific resistance detection or research-based profiling
- LNZ 30 µg – Standard dose for routine susceptibility testing
-
Broad Spectrum Gram-Positive Coverage
- Effective against Staphylococcus spp., Enterococcus spp., and Streptococcus spp.
-
CLSI and EUCAST Compliant
- Results can be interpreted against global susceptibility breakpoints
-
Accurate Zone Inhibition Measurement
- Helps differentiate susceptible, intermediate, or resistant strains
-
High-Quality Manufacturing
- Each disc contains a precise dose absorbed onto high-purity paper
- Supplied in packs of 5 cartridges × 50 discs = 250 discs
-
Cold-Chain Friendly
- Refrigerated storage (2–8°C) ensures disc stability and potency
Applications
- Routine antimicrobial susceptibility testing in clinical microbiology
- Resistance surveillance and stewardship programs
- MRSA and VRE detection
- Research studies on oxazolidinone antibiotics
- Therapeutic guidance for infectious disease treatment teams
Product Specifications
| Attribute | Specification |
|---|---|
| Antibiotic | Linezolid (LNZ) |
| Concentration | 10 µg or 30 µg per disc |
| Disc Diameter | 6 mm |
| Disc Material | High-absorbency filter paper |
| Product Format | 5 × 50 discs/cartridge (250 discs total) |
| Storage Conditions | 2–8°C (refrigerated) |
| Shelf Life | Typically 24 months from manufacture |
| Compliance | CLSI M100, EUCAST |
| Use | In vitro diagnostic use (IVD) |
Ordering Information
| Product Description | Reference Code | Packaging |
|---|---|---|
| Linezolid LNZ 30 µg – 5 × 50 discs | 9136 | 250 discs per pack |
| Linezolid LNZ 10 µg – 5 × 50 discs | 9155 | 250 discs per pack |
For best results, use with Mueller-Hinton Agar and refer to CLSI M100 or EUCAST breakpoint tables for interpretation of inhibition zones.
Liofilchem® Linezolid Susceptibility Discs (10 µg and 30 µg) offer an accurate, validated, and efficient means for determining bacterial susceptibility to linezolid, a critical agent in the treatment of multidrug-resistant Gram-positive infections. With precise dosing, CLSI/EUCAST compliance, and ISO-certified manufacturing, they are indispensable tools for clinical laboratories, resistance surveillance programs, and infectious disease research.
- Drug Concentration: 30 µg 10 µg




 0
0