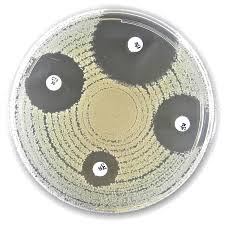
Liofilchem® Fosfomycin FOS Antimicrobial Susceptibility Discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Fosfomycin FOS Discs (200 µg) are high-quality antimicrobial susceptibility testing (AST) discs designed for in vitro use with the Kirby-Bauer disk diffusion method on Mueller-Hinton agar supplemented with glucose-6-phosphate (G6P). Each disc contains 200 micrograms of Fosfomycin, a phosphonic acid derivative antibiotic with bactericidal activity against a broad range of Gram-negative and some Gram-positive bacteria. These discs are manufactured under ISO 9001 and ISO 13485 standards, ensuring accuracy, sterility, and consistency for clinical diagnostics and resistance surveillance.
Key Features
- High-potency formulation: Each disc contains 200 µg of Fosfomycin to ensure accurate zone interpretation in accordance with international guidelines.
- Broad-spectrum activity: Effective against Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae, Proteus mirabilis, Enterobacter spp., and multidrug-resistant uropathogens.
- CLSI and EUCAST compliant: Designed for use with standardized AST methods that include glucose-6-phosphate supplementation.
- Sterile and stable: Packaged in sealed cartridges or canisters with desiccant to preserve efficacy and extend shelf life.
- Color-coded labeling: Enables fast and accurate disc identification in busy laboratories.
Applications and Use Cases
1. Clinical Microbiology
Used in hospital and diagnostic laboratories to:
-
Determine the susceptibility of:
- Escherichia coli, especially in uncomplicated urinary tract infections (UTIs)
- Extended-spectrum β-lactamase (ESBL)-producing Enterobacterales
- Carbapenem-resistant Klebsiella pneumoniae (CRE)
- Enterococcus faecalis and Staphylococcus saprophyticus
-
Support antibiotic selection in:
- Community- and hospital-acquired UTIs
- Infections caused by multidrug-resistant uropathogens
2. Antimicrobial Stewardship and Surveillance
- Monitor Fosfomycin resistance patterns, especially in urology and nephrology patient populations.
- Track susceptibility in ESBL and CRE isolates as part of infection control programs.
3. Research and Development
- Evaluate Fosfomycin efficacy against biofilm-forming or resistant strains.
- Study the synergy of Fosfomycin in combination therapies (e.g., with aminoglycosides or carbapenems).
- Test novel formulations or delivery systems in pharmaceutical development.
Use Instructions
- Prepare Mueller-Hinton agar supplemented with 25 µg/mL glucose-6-phosphate (G6P) as required for Fosfomycin testing.
- Standardize bacterial suspension to 0.5 McFarland turbidity.
- Inoculate the plate uniformly with a sterile swab.
- Apply one Fosfomycin disc (200 µg) using sterile forceps or a disc dispenser.
- Incubate at 35 ± 2 °C for 16–18 hours.
- Measure the diameter of the inhibition zone in millimeters.
- Interpret using the latest CLSI or EUCAST breakpoints, considering organism-specific criteria (e.g., E. coli from urine).
Storage and Shelf Life
- Storage Conditions: 2–8 °C (refrigerated), in original packaging with desiccant.
- Shelf Life: 24–36 months from the date of manufacture when properly stored.
Liofilchem® Fosfomycin FOS Discs (200 µg) provide a standardized and effective tool for detecting bacterial susceptibility to Fosfomycin, particularly for urinary pathogens and multidrug-resistant Enterobacterales. Backed by ISO-certified manufacturing and aligned with CLSI/EUCAST guidelines, these discs support clinical decision-making, resistance tracking, and antibiotic stewardship. They are an essential addition to the modern microbiology laboratory's toolkit for combating antimicrobial resistance.
- Drug Concentration: 50 µg 100 µg 200 µg




 0
0