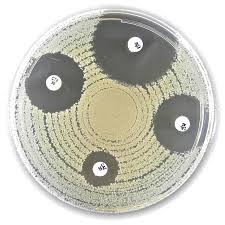
Liofilchem® Ceftazidime CAZ Antimicrobial Susceptibility Discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Ceftazidime (CAZ) Antimicrobial Susceptibility Discs are high-quality discs designed for in vitro antibiotic susceptibility testing (AST) of bacterial isolates. These discs are impregnated with Ceftazidime (CAZ) at concentrations of 10 µg and 30 µg, enabling laboratories to determine the susceptibility or resistance of bacterial pathogens to this broad-spectrum third-generation cephalosporin.
Ceftazidime is a β-lactam antibiotic with strong activity against Gram-negative bacteria, including Pseudomonas aeruginosa and Enterobacteriaceae. The susceptibility discs are compliant with international standards such as EUCAST (European Committee on Antimicrobial Susceptibility Testing) and CLSI (Clinical and Laboratory Standards Institute).
Product Specifications
- Product Name: Liofilchem® Ceftazidime (CAZ) Antimicrobial Susceptibility Discs
- Antibiotic: Ceftazidime (CAZ)
- Disc Strengths: 10 µg and 30 µg
- Application: Antimicrobial Susceptibility Testing (AST)
- Disc Type: Paper discs impregnated with antibiotic
- Intended Use: In vitro testing of bacterial susceptibility
- Organism Spectrum: Primarily Gram-negative bacteria, including multidrug-resistant strains
Key Features & Benefits
1. Accurate & Reliable AST Results
- Provides quantifiable zone of inhibition for determining bacterial susceptibility
- Helps guide antibiotic therapy selection for infectious diseases
- Ensures quality control in microbiology laboratories
2. Standardized for Clinical Use
- Conforms to EUCAST and CLSI breakpoints
- Compatible with Kirby-Bauer disc diffusion method
- Ensures reproducible and standardized test conditions
3. Broad Spectrum of Activity
- Highly effective against Gram-negative pathogens, including:
- Pseudomonas aeruginosa
- Escherichia coli
- Klebsiella pneumoniae
- Enterobacter spp.
- Proteus spp.
- Citrobacter spp.
4. Two Strength Variants (10 µg & 30 µg)
- 10 µg disc: Often used for pharmacodynamic studies and research
- 30 µg disc: Standard for clinical diagnostics and AST
5. Quality Manufacturing & Storage
- Sterile and high-purity discs for precise results
- Long shelf life with optimal storage conditions (2-8°C)
- Delivered in hermetically sealed cartridges to maintain stability
Use Cases
1. Clinical Microbiology & Infectious Disease Diagnosis
Hospitals and diagnostic laboratories use Ceftazidime (CAZ) susceptibility discs for routine bacterial culture analysis, ensuring effective antibiotic therapy decisions for infections such as:
- Nosocomial infections (hospital-acquired infections)
- Sepsis and bloodstream infections
- Urinary tract infections (UTIs) caused by multidrug-resistant bacteria
- Pneumonia (including ventilator-associated pneumonia)
- Wound and surgical site infections
- Cystic fibrosis-related lung infections caused by Pseudomonas aeruginosa
2. Antibiotic Stewardship Programs
- Helps monitor resistance trends in Gram-negative bacteria
- Supports rational antibiotic prescribing
- Assists in identifying multi-drug resistant (MDR) pathogens
3. Pharmaceutical & Research Laboratories
- Quality control in antibiotic development
- Used in antibiotic resistance surveillance studies
- Research on extended-spectrum β-lactamase (ESBL)-producing bacteria
4. Veterinary Microbiology
- Determines antimicrobial susceptibility of animal bacterial pathogens
- Assists in antibiotic selection for veterinary infections
5. Environmental & Industrial Microbiology
- Applied in food safety testing for contamination by antibiotic-resistant bacteria
- Used in water and soil microbiology to assess antibiotic resistance spread
Testing Methodology: Kirby-Bauer Disc Diffusion
The Kirby-Bauer method is widely used for Ceftazidime (CAZ) susceptibility testing:
- Prepare the inoculum: A bacterial suspension is adjusted to 0.5 McFarland standard.
- Plate inoculation: The bacterial culture is spread evenly on Mueller-Hinton agar (MHA).
- Disc placement: The Ceftazidime disc (10 µg or 30 µg) is placed onto the agar.
- Incubation: The plate is incubated at 35-37°C for 16-18 hours.
- Zone measurement: The diameter of the inhibition zone is measured and interpreted using CLSI/EUCAST breakpoints.
Clinical Significance of Ceftazidime (CAZ) in AST
- Effective against severe Gram-negative infections, especially in hospitalized and immunocompromised patients.
- Resistance mechanisms: Some bacteria produce β-lactamases (ESBLs, AmpC, or carbapenemases), reducing Ceftazidime effectiveness.
- Combination therapy consideration: Often used with β-lactamase inhibitors (e.g., Ceftazidime/Avibactam) for treating resistant strains.
Breakpoints & Interpretation (EUCAST/CLSI)
| Disc Strength | Zone Diameter (mm) | Interpretation (CLSI) |
|---|---|---|
| 30 µg | ≥ 21 mm | Susceptible (S) |
| 30 µg | 18-20 mm | Intermediate (I) |
| 30 µg | ≤ 17 mm | Resistant (R) |
Note: Breakpoints may vary based on guidelines and bacterial species.
Liofilchem® Ceftazidime (CAZ) Antimicrobial Susceptibility Discs (10 µg & 30 µg) provide accurate, reliable, and standardized testing for Gram-negative bacterial infections. Their essential role in clinical diagnostics, antimicrobial resistance surveillance, and pharmaceutical research makes them a crucial tool for microbiologists and healthcare professionals.
- Drug Concentration: 30 µg 10 µg




 0
0