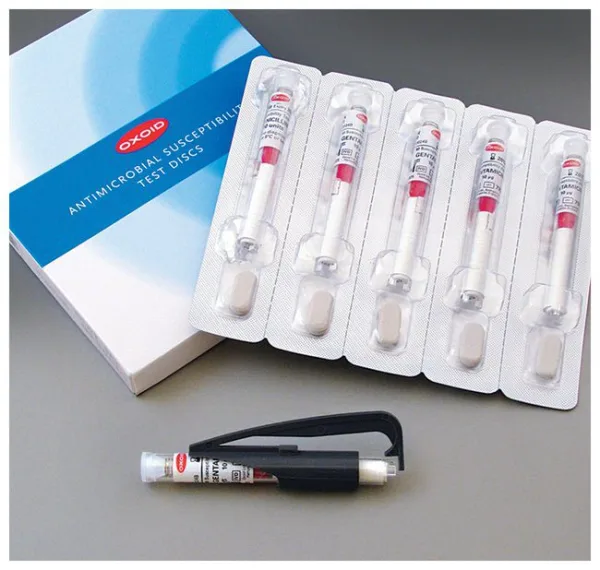
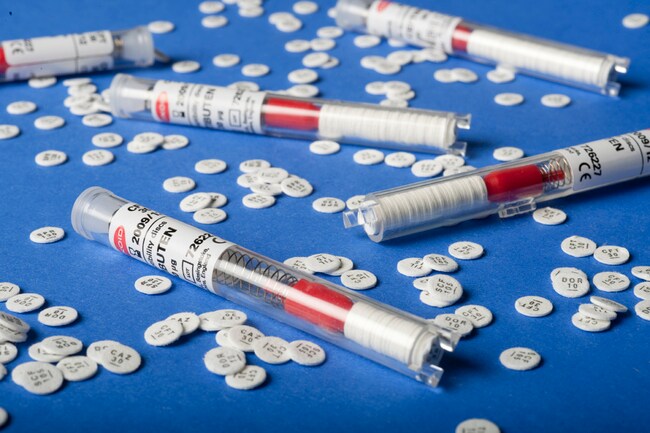
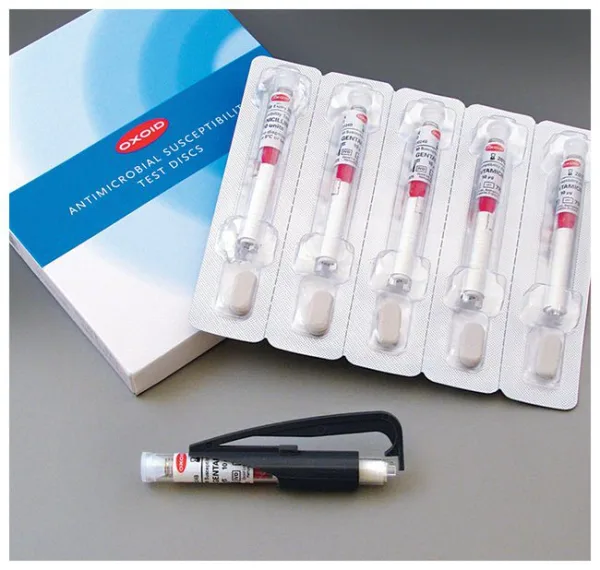
Thermo Scientific™ Oxoid™ Tigecycline TGC Antimicrobial Susceptibility discs, 15 μg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Thermo Fisher Scientific™, Oxoid |
| Product Grade | Microbiology grade |
The Thermo Scientific™ Oxoid™ Tigecycline Antimicrobial Susceptibility Discs are standardized 6 mm paper discs pre-impregnated with 15 μg of tigecycline, a broad-spectrum glycylcycline antibiotic. These discs are designed for manual antimicrobial susceptibility testing (AST) using the Kirby-Bauer disc diffusion method, allowing for accurate evaluation of bacterial sensitivity to tigecycline in clinical microbiology.
Tigecycline is effective against a wide range of multidrug-resistant organisms, including carbapenem-resistant Enterobacteriaceae (CRE), MRSA, VRE, and Acinetobacter baumannii. These discs are widely used in healthcare settings for monitoring antimicrobial resistance and guiding therapy decisions in serious infections.
Each disc is dispensed via a shatterproof spring-loaded cartridge for easy and sterile application. The cartridges are individually foil-sealed with desiccant, ensuring moisture protection (<2%) and long-term stability.
Key Features
- Contains 15 μg of tigecycline per 6 mm disc
- Designed for detection of resistance in multidrug-resistant organisms (MDROs)
- Supplied in sealed spring-loaded cartridges for ease of use and sterility
- Each cartridge holds 50 discs and is desiccant-sealed to maintain disc integrity
- Complies with international AST standards: CLSI, EUCAST, BSAC, and SFM
- Enables classification of isolates as susceptible, intermediate, or resistant
- For in vitro diagnostic use only
Applications
- Susceptibility testing for tigecycline in Enterobacteriaceae, Staphylococcus aureus, Enterococcus spp., and Acinetobacter
- Resistance profiling in multidrug-resistant infections
- Clinical decision-making for severe or healthcare-associated infections
- AST screening in public health laboratories and hospital microbiology departments
- Monitoring of emerging tigecycline resistance
Packaging Format
| Component | Details |
|---|---|
| Disc Diameter | 6 mm |
| Discs per Cartridge | 50 |
| Cartridges per Pack | 5 |
| Total Discs per Pack | 250 |
| Packaging Type | Foil blister with desiccant |
Specifications
| Parameter | Details |
|---|---|
| Product Type | Antimicrobial Susceptibility Discs |
| Antibiotic Code | TGC |
| Antimicrobial Agent | Tigecycline |
| Concentration | 15 μg |
| Compliance Standards | CLSI, EUCAST, BSAC, SFM |
| Test Method | Kirby-Bauer Disc Diffusion |
| Intended Use | In vitro diagnostic use |
Oxoid™ Tigecycline Antimicrobial Susceptibility Discs provide a reliable, standardized method for detecting resistance to tigecycline in clinically significant bacterial pathogens. Backed by global AST standards and packaged for precision and stability, these discs are a critical tool in the fight against antimicrobial resistance in modern microbiology laboratories.




 0
0