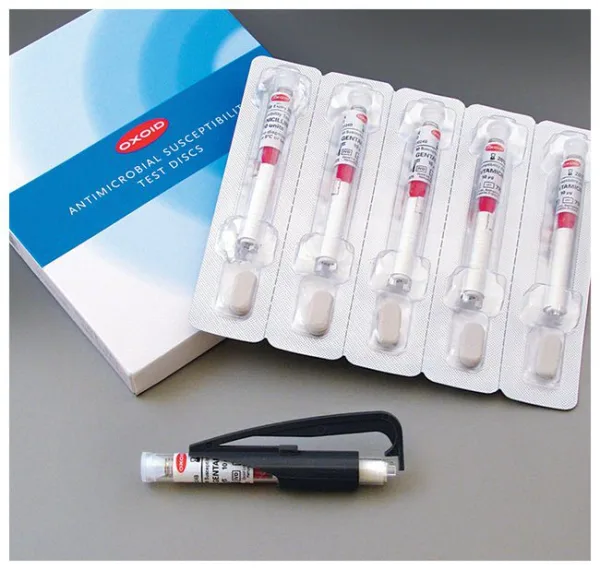
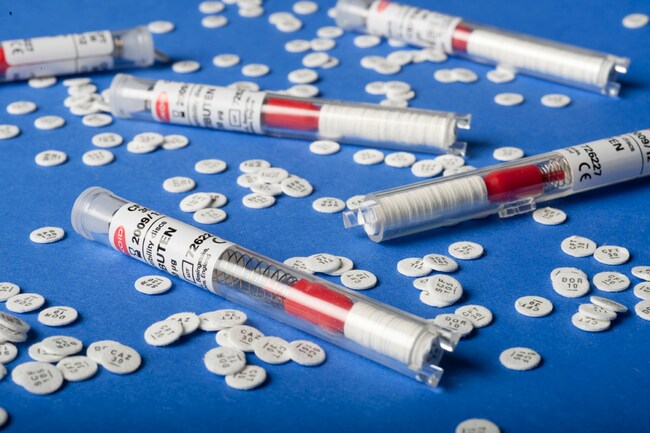
Thermo Scientific™ Oxoid™ Piperacillin-tazobactam TZP, Antimicrobial Susceptibility Discs, 110μg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Thermo Fisher Scientific™, Oxoid |
| Product Grade | Microbiology grade |
The Thermo Scientific™ Oxoid™ Piperacillin/Tazobactam Antimicrobial Susceptibility Discs are precision-engineered tools used in manual antimicrobial susceptibility testing (AST), particularly by the Kirby-Bauer disc diffusion method. Each disc contains 110 μg of a fixed combination of Piperacillin, a broad-spectrum ureidopenicillin, and Tazobactam, a β-lactamase inhibitor, enabling reliable detection of bacterial resistance mechanisms such as extended-spectrum β-lactamases (ESBLs).
Designed to deliver clear, reproducible zone-of-inhibition results, these discs support semi-quantitative in vitro diagnostics by providing key visual indicators of antimicrobial activity. The discs are housed in spring-loaded, shatterproof cartridges with individually sealed desiccant barriers to maintain optimal moisture levels (<2%), ensuring consistent potency and long-term stability.
These discs are ideal for use in clinical microbiology laboratories, antimicrobial resistance surveillance programs, and infection control workflows.
Key Features and Benefits
- Potent Dual-Agent Disc (110 μg): Combines 100 μg Piperacillin and 10 μg Tazobactam for synergistic inhibition of resistant Gram-negative pathogens.
- Ease-of-Use: Convenient shatterproof cartridges with easy-to-read labeling and end-of-cartridge indicators streamline testing workflows.
- Stable & Reliable: Moisture-controlled cartridges ensure extended shelf life and consistent zone diameters.
- Diagnostic Utility: Enables detection of β-lactamase-producing bacteria and supports resistance mechanism profiling (e.g., ESBL, AmpC).
- Compliant with Global Standards: Conforms to CLSI, EUCAST, and BSAC guidelines for antimicrobial susceptibility testing.
- Versatile Compatibility: Suitable for use with a wide range of Enterobacteriaceae, Pseudomonas spp., and Acinetobacter spp.
Intended Use
For in vitro diagnostic use in determining the susceptibility of bacteria to Piperacillin/Tazobactam. Recommended for use in laboratories performing disk diffusion susceptibility testing on pure agar-grown cultures.
Applications
- Hospital-acquired and multidrug-resistant infection surveillance
- Gram-negative resistance detection (e.g., Pseudomonas, Enterobacteriaceae)
- Microbiology laboratories and clinical diagnostic testing
- Antimicrobial stewardship programs
Technical Specifications
| Attribute | Description |
|---|---|
| Product Code | CT0725B |
| Antibiotic Code | TZP |
| Antimicrobial Agent | Piperacillin/Tazobactam |
| Disc Potency | 110 μg (100 μg Piperacillin + 10 μg Tazobactam) |
| Format | 5 cartridges per pack |
| Discs per Cartridge | 50 |
| Packaging Type | Spring-loaded cartridges with desiccant |
| Compliance | CLSI |
| Test Type | Manual Antimicrobial Susceptibility Testing (AST) |
| Unit Size | Each (Pack of 5 cartridges) |
Note: This product is for professional use only. Not all configurations may be available in all territories. Contact your local distributor for availability and regulatory status in your region.




 0
0