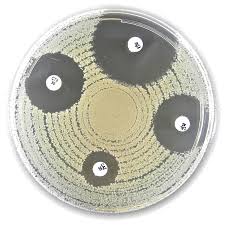
The Liofilchem® Optochin Test is a diagnostic tool for the identification of Streptococcus pneumoniae by differentiating it from other alpha-hemolytic streptococci based on their susceptibility to optochin (ethylhydrocupreine hydrochloride).
Key Features
- High Specificity:
- Differentiates pneumococci from other alpha-hemolytic streptococci using optochin sensitivity.
- Ease of Use:
- Paper discs are pre-impregnated with 5 µg of optochin for direct application on agar plates.
- Quick Results:
- Results are available within 18-24 hours of incubation.
- Validated Protocol:
- Quality control ensures reliable and consistent results.
Specifications
Kit Contents
- Discs: 2 cartridges, each containing 50 optochin-impregnated discs.
- Packaging: Individually sealed in a foil blister pack with a desiccant.
Key Characteristics
| Parameter | Details |
|---|
| Disc Composition | 5 µg of optochin per disc |
| Storage Temperature | -20°C to +8°C |
| Shelf Life | 2 years |
| After Opening | Use within 7 days when stored at 2-8°C |
| Incubation Conditions | 35 ± 2°C in 5% CO₂ atmosphere for 18-24 hours |
Performance
Interpretation of Results
| Inhibition Zone Diameter | Interpretation |
|---|
| ≥ 14 mm | Sensitive (S): Presumptive S. pneumoniae |
| < 14 mm or None | Resistant (R): Not S. pneumoniae |
Control Strains
| Microorganism | ATCC® | Expected Results |
|---|
| Streptococcus pneumoniae | 6305 | Sensitive, zone ≥ 14 mm |
| Streptococcus pyogenes | 19615 | Resistant, no inhibition zone |
Applications
- Clinical Diagnostics:
- Differentiates S. pneumoniae from other alpha-hemolytic streptococci in patient samples.
- Research Laboratories:
- Supports pneumococcal studies and epidemiological research.
- Educational Use:
- Demonstrates bacterial identification techniques in microbiology courses.
Usage Guidelines
Test Procedure
- Bring the product to room temperature before use.
- Inoculate a blood agar plate with the test organism.
- Apply an Optochin Test disc using sterile forceps.
- Incubate the plate at 35 ± 2°C for 18-24 hours in 5% CO₂.
- Measure the inhibition zone diameter using a ruler or caliper.
Storage and Precautions
- Storage:
- Store unopened cartridges at -20°C/+8°C.
- Opened discs must be stored at 2-8°C and used within 7 days.
- Precautions:
- For in vitro diagnostic use only.
- Handle all specimens as potentially infectious.
- Ensure laboratory equipment is calibrated and maintained.
Advantages
- Accurate Differentiation:
- Proven effectiveness in identifying S. pneumoniae.
- Convenience:
- Compact and pre-prepared for immediate use.
- Cost-Effective:
- Reduces the need for advanced molecular diagnostics.
Warnings
- Optochin susceptibility is a presumptive test and requires confirmation via biochemical or serological testing.
- CO₂ concentration during incubation affects results; ensure optimal conditions.
- Occasional optochin-resistant S. pneumoniae strains may lead to false-negative results.
References
- Public Health England (2018). Optochin Test. UK Standards for Microbiology Investigations. TP 25 Issue 4.
- Lennette EH, Balows A, Hausler WJ, Truant JP (1980). Manual of Clinical Microbiology. 3rd ed. ASM, Washington, D.C.
https://www.labmartgh.com/shop/product/9501-liofilchem-r-optochin-test-discs-10330
Product Specification not found.




 0
0