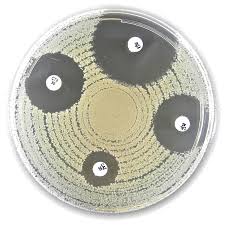
Liofilchem® Ampliclox ACL Antimicrobial Susceptibility Discs, 30 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Liofilchem® Ampliclox (ACL) Antimicrobial Susceptibility Discs, containing a combination of Ampicillin (25 µg) and Cloxacillin (5 µg), are used for testing the susceptibility of bacterial pathogens to this antibiotic combination. These discs are essential tools in clinical microbiology for evaluating the efficacy of Ampliclox, a beta-lactam combination commonly prescribed for Gram-positive and Gram-negative bacterial infections.
Key Features
- Dual-Drug Combination:
- Ampicillin (25 µg): Broad-spectrum beta-lactam antibiotic effective against Gram-positive and some Gram-negative bacteria.
- Cloxacillin (5 µg): A beta-lactamase-resistant penicillin effective against beta-lactamase-producing Gram-positive cocci, such as Staphylococcus aureus.
- High Precision: Designed to evaluate the synergistic activity of Ampliclox against bacterial infections, particularly in cases where beta-lactamase production is a concern.
- Standardized Testing: Manufactured following CLSI and EUCAST guidelines, ensuring accuracy and reproducibility.
- Ready-to-Use: Pre-dosed discs for ease of application and consistent results.
Applications
- Clinical Microbiology:
- Used to test bacterial isolates from clinical specimens (e.g., blood, respiratory samples, wound swabs, urine) for susceptibility to the Ampliclox combination.
- Effective for infections caused by beta-lactamase-producing Staphylococcus spp. and Streptococcus spp.
- Antimicrobial Resistance Monitoring:
- Tracks resistance patterns in Gram-positive pathogens, especially in Staphylococcus aureus and Enterococcus spp.
- Research Laboratories:
- Assists in studying the synergistic effects of ampicillin and cloxacillin against bacterial pathogens.
Test Procedure
- Preparation:
- Prepare Mueller-Hinton Agar (MHA) according to the standard protocol.
- Adjust the bacterial suspension to a 0.5 McFarland standard.
- Application:
- Using sterile forceps or a disc dispenser, place the ACL 30 µg disc on the agar surface previously inoculated with the bacterial suspension.
- Ensure even contact between the disc and the agar.
- Incubation:
- Incubate the plates at 35 ± 2°C for 16–20 hours in an aerobic environment.
- Result Interpretation:
- Measure the diameter of the inhibition zone in millimeters (mm).
- Compare the zone size with breakpoint tables provided by CLSI or EUCAST to determine susceptibility (S), intermediate (I), or resistance (R).
Quality Control
The performance of ACL discs is validated using the following quality control strains:
- Escherichia coli ATCC® 25922 – Expected zone diameter within established ranges for Ampliclox activity.
- Staphylococcus aureus ATCC® 25923 – Ensures effectiveness against beta-lactamase-producing staphylococci.
Storage and Stability
- Store discs at 2–8°C in their original packaging to protect from moisture and light.
- Use before the expiration date printed on the label.
- Avoid prolonged exposure to high temperatures or humidity, which may affect the potency of the discs.
Ordering Information
- Ref. ACL030 – Pack of 50 discs in cartridges.
- Ref. ACL060 – Pack of 100 discs in cartridges.
Precautions
- For in vitro diagnostic use (IVD) only.
- Handle discs with sterile forceps to prevent contamination.
- Dispose of used discs and culture plates in accordance with institutional biosafety and biohazard guidelines.
Regulatory Compliance
- Manufactured in compliance with ISO 11133, CLSI, and EUCAST standards for antimicrobial susceptibility testing.
References
- Clinical and Laboratory Standards Institute (CLSI) guidelines.
- EUCAST breakpoint interpretations.
- Manufacturer’s technical documentation for Liofilchem® ACL discs.
Key Benefits
- Enhanced Activity: Combines the spectrum of ampicillin with the beta-lactamase resistance of cloxacillin.
- Broad Clinical Application: Effective against a wide range of Gram-positive and Gram-negative pathogens.
- Accurate Results: Meets stringent quality control parameters for reliable susceptibility testing.
4o
The Liofilchem® Ampliclox (ACL) Antimicrobial Susceptibility Discs, containing a combination of Ampicillin (25 µg) and Cloxacillin (5 µg), are used for testing the susceptibility of bacterial pathogens to this antibiotic combination. These discs are essential tools in clinical microbiology for evaluating the efficacy of Ampliclox, a beta-lactam combination commonly prescribed for Gram-positive and Gram-negative bacterial infections.
Key Features
- Dual-Drug Combination:
- Ampicillin (25 µg): Broad-spectrum beta-lactam antibiotic effective against Gram-positive and some Gram-negative bacteria.
- Cloxacillin (5 µg): A beta-lactamase-resistant penicillin effective against beta-lactamase-producing Gram-positive cocci, such as Staphylococcus aureus.
- High Precision: Designed to evaluate the synergistic activity of Ampliclox against bacterial infections, particularly in cases where beta-lactamase production is a concern.
- Standardized Testing: Manufactured following CLSI and EUCAST guidelines, ensuring accuracy and reproducibility.
- Ready-to-Use: Pre-dosed discs for ease of application and consistent results.
Applications
- Clinical Microbiology:
- Used to test bacterial isolates from clinical specimens (e.g., blood, respiratory samples, wound swabs, urine) for susceptibility to the Ampliclox combination.
- Effective for infections caused by beta-lactamase-producing Staphylococcus spp. and Streptococcus spp.
- Antimicrobial Resistance Monitoring:
- Tracks resistance patterns in Gram-positive pathogens, especially in Staphylococcus aureus and Enterococcus spp.
- Research Laboratories:
- Assists in studying the synergistic effects of ampicillin and cloxacillin against bacterial pathogens.
Test Procedure
- Preparation:
- Prepare Mueller-Hinton Agar (MHA) according to the standard protocol.
- Adjust the bacterial suspension to a 0.5 McFarland standard.
- Application:
- Using sterile forceps or a disc dispenser, place the ACL 30 µg disc on the agar surface previously inoculated with the bacterial suspension.
- Ensure even contact between the disc and the agar.
- Incubation:
- Incubate the plates at 35 ± 2°C for 16–20 hours in an aerobic environment.
- Result Interpretation:
- Measure the diameter of the inhibition zone in millimeters (mm).
- Compare the zone size with breakpoint tables provided by CLSI or EUCAST to determine susceptibility (S), intermediate (I), or resistance (R).
Quality Control
The performance of ACL discs is validated using the following quality control strains:
- Escherichia coli ATCC® 25922 – Expected zone diameter within established ranges for Ampliclox activity.
- Staphylococcus aureus ATCC® 25923 – Ensures effectiveness against beta-lactamase-producing staphylococci.
Storage and Stability
- Store discs at 2–8°C in their original packaging to protect from moisture and light.
- Use before the expiration date printed on the label.
- Avoid prolonged exposure to high temperatures or humidity, which may affect the potency of the discs.
Precautions
- For in vitro diagnostic use (IVD) only.
- Handle discs with sterile forceps to prevent contamination.
- Dispose of used discs and culture plates in accordance with institutional biosafety and biohazard guidelines.
Regulatory Compliance
- Manufactured in compliance with ISO 11133, CLSI, and EUCAST standards for antimicrobial susceptibility testing.
References
- Clinical and Laboratory Standards Institute (CLSI) guidelines.
- EUCAST breakpoint interpretations.
- Manufacturer’s technical documentation for Liofilchem® ACL discs.
Key Benefits
- Enhanced Activity: Combines the spectrum of ampicillin with the beta-lactamase resistance of cloxacillin.
- Broad Clinical Application: Effective against a wide range of Gram-positive and Gram-negative pathogens.
- Accurate Results: Meets stringent quality control parameters for reliable susceptibility testing




 0
0