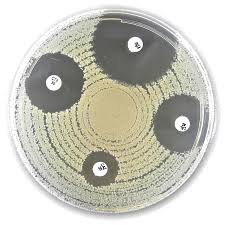
Liofilchem® Oxytetracycline OT Antimicrobial Susceptibility Discs, 30 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Oxytetracycline (OT) Antimicrobial Susceptibility Discs (30 µg) are standardized, high-quality paper discs used for in vitro assessment of bacterial susceptibility to oxytetracycline, a broad-spectrum tetracycline-class antibiotic. These discs are designed for Kirby-Bauer disk diffusion method and are suitable for testing various Gram-positive and Gram-negative bacteria of clinical, veterinary, and environmental relevance.
Oxytetracycline interferes with bacterial protein synthesis by binding to the 30S ribosomal subunit, inhibiting aminoacyl-tRNA binding. Liofilchem manufactures these discs under ISO 9001 and ISO 13485 quality standards to ensure consistency, sterility, and accuracy in susceptibility testing.
Key Features
- Standardized concentration: Each disc is impregnated with 30 µg of oxytetracycline for reliable zone of inhibition measurement.
- Broad-spectrum activity: Effective against organisms such as E. coli, Salmonella spp., Pasteurella spp., Staphylococcus spp., and Streptococcus spp.
- Validated for clinical and veterinary use: Applicable for both human and animal bacterial pathogen susceptibility profiling.
- CLSI and EUCAST compatible: Suitable for laboratories following international AST interpretation standards.
- Sterile and shelf-stable: Packaged in moisture-protected cartridges or canisters with desiccants to preserve disc integrity.
- Color-coded labeling: Ensures quick identification and traceability in multi-disc workflows.
Applications and Use Cases
1. Clinical Microbiology
- Evaluate oxytetracycline susceptibility in enteric pathogens, respiratory isolates, and soft tissue infections.
- Part of routine AST panels for tetracycline-class antibiotics.
2. Veterinary Diagnostics
- Widely used for AST in livestock and poultry pathogens such as E. coli, Salmonella, Pasteurella, and Staphylococcus pseudintermedius.
- Supports antibiotic stewardship in animal health management.
3. Food Safety and Environmental Monitoring
- Resistance monitoring in zoonotic pathogens isolated from food, water, and animal waste.
4. Pharmaceutical and Academic Research
- Comparative evaluation with other tetracyclines (e.g., tetracycline, doxycycline).
- Research on resistance mechanisms (e.g., tet genes) in Gram-negative and Gram-positive isolates.
Use Instructions
- Medium: Mueller-Hinton Agar (MHA) is recommended.
- Inoculum preparation: Adjust bacterial suspension to 0.5 McFarland standard.
- Inoculation: Evenly spread the suspension on agar surface using a sterile swab.
- Disc application: Place a 30 µg Oxytetracycline disc on the plate using sterile forceps or a disc dispenser.
-
Incubation:
- Temperature: 35 ± 2 °C
- Duration: 16–18 hours under aerobic conditions
-
Interpretation:
- Measure zone diameter (in mm) and compare to CLSI or EUCAST interpretive criteria.
- Susceptibility categories: Susceptible (S), Intermediate (I), Resistant (R).
Technical Specifications
| Specification | Detail |
|---|---|
| Antibiotic | Oxytetracycline (OT) |
| Disc Potency | 30 µg |
| Disc Diameter | 6 mm |
| Disc Material | Filter paper, sterile |
| Packaging | Cartridges (50 discs) / Canisters (250 discs) |
| Storage Conditions | 2–8 °C (with desiccant, sealed container) |
| Shelf Life | 24–36 months from date of manufacture |
Quality Control
Quality-assured using standard reference strains:
| Control Organism | Expected Zone Range |
|---|---|
| Escherichia coli ATCC® 25922 | Within CLSI range |
| Staphylococcus aureus ATCC® 25923 | Within CLSI range |
| Salmonella enterica (clinical isolate) | Confirmed activity |
Liofilchem® Oxytetracycline OT Discs (30 µg) provide a dependable, validated method for assessing bacterial susceptibility to a commonly used tetracycline antibiotic. Trusted in both clinical and veterinary sectors, they support effective antimicrobial therapy decisions, antibiotic resistance surveillance, and comparative antimicrobial research. Manufactured to international quality standards, these discs ensure accurate and reproducible results across microbiology testing environments.




 0
0