Liofilchem® Mycoplasma System Plus for 20 Tests
Catalog No :
CAS Number :
Brand :
In Stock
Count, Identification and Susceptibility Testing of urogenital mycoplasmas and Candida spp./Trichomonas vaginalis directly from Cinical Specimens
Specifications:
| Application | Clinical microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Microbial ID System |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Mycoplasma System Plus is an advanced diagnostic tool for the counting, identification, and susceptibility testing of urogenital mycoplasmas (Ureaplasma spp., Mycoplasma hominis) and the direct identification of Candida spp. and Trichomonas vaginalis from clinical specimens. Designed for efficiency and accuracy, this system provides comprehensive results in 24–48 hours with clear, well-defined colorimetric changes.
Key Features and Benefits
- Comprehensive Testing:
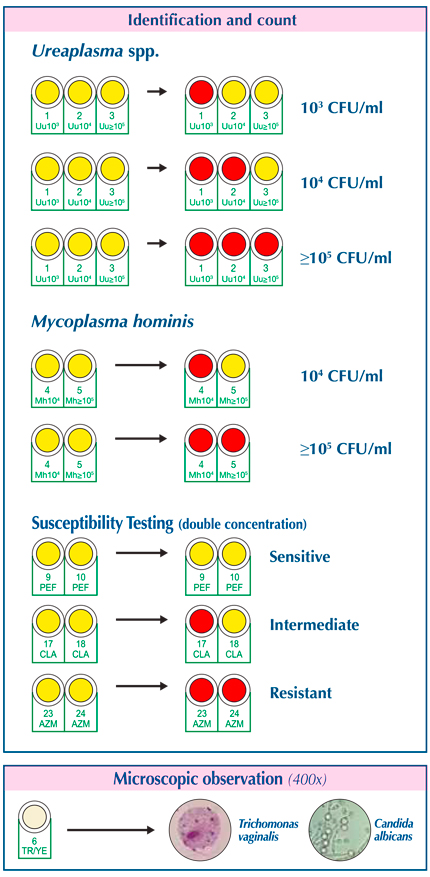
- Counts and Identifies Urogenital Mycoplasmas:
- Ureaplasma spp.
- Mycoplasma hominis
- Susceptibility Testing for Mycoplasmas:
- Double-concentration testing for antibiotics:
- Tetracycline, Pefloxacin, Ofloxacin
- Doxycycline, Erythromycin, Clarithromycin
- Minocycline, Clindamycin, Azithromycin
- Double-concentration testing for antibiotics:
- Microscopic Identification:
- Direct observation of:
- Candida spp.
- Trichomonas vaginalis
- Direct observation of:
- Counts and Identifies Urogenital Mycoplasmas:
- Wide Range of Clinical Specimens:
- Compatible with:
- Vaginal swabs
- Urethral swabs
- Rectal swabs
- Seminal fluid
- Compatible with:
- Efficiency:
- Direct Testing: Reduces time and labor in diagnostic workflows.
- Fast Results: Available within 24–48 hours.
- Ease of Use: Clear color changes for straightforward interpretation.
- Enhanced Workflow:
- Automatic Reading: System-compatible for automated result reading.
- Auxiliary Products: Supports transport and testing with:
- Mycoplasma Transport Broth (20x6 mL, Ref. 20158)
- Vaseline Oil Droppers (10x10 mL, Ref. 87006)
Applications
- Clinical Diagnostics:
- Rapid diagnosis of urogenital infections caused by:
- Ureaplasma spp.
- Mycoplasma hominis
- Candida spp. and Trichomonas vaginalis
- Evaluation of antimicrobial resistance in urogenital mycoplasmas.
- Rapid diagnosis of urogenital infections caused by:
- Public Health Monitoring:
- Essential for monitoring sexually transmitted infections (STIs) and antimicrobial resistance patterns.
- Reproductive Health:
- Assessment of microorganisms linked to infertility, pelvic inflammatory disease (PID), and other urogenital conditions.
Test Procedure
- Sample Preparation:
- Collect clinical specimens (vaginal, urethral, rectal swabs, or seminal fluid) using appropriate sterile techniques.
- Panel Inoculation:
- Inoculate the panel wells with the prepared specimen.
- Incubation:
- Incubate the panel at 36 ± 1°C for 24–48 hours.
- Interpretation:
- Observe color changes in the wells for microbial growth and identification.
- Evaluate susceptibility based on growth patterns in antibiotic-containing wells.
Specifications and Packaging
| Product | Description | Tests/Volume | Reference |
|---|---|---|---|
| Mycoplasma System Plus | Mycoplasma diagnostic panel | 20 tests | 72592 |
| Vaseline Oil Droppers | Seals panel wells during incubation | 10x10 mL | 87006 |
| Mycoplasma Transport Broth | Preserves specimen viability before testing | 20x6 mL | 20158 |
Clinical Significance
- Diagnosis of Urogenital Infections:
- Detects infections caused by mycoplasmas, Candida spp., and Trichomonas vaginalis, which are associated with:
- Pelvic inflammatory disease (PID)
- Vaginosis and vaginitis
- Urethritis and prostatitis
- Infertility and preterm labor
- Detects infections caused by mycoplasmas, Candida spp., and Trichomonas vaginalis, which are associated with:
- Antimicrobial Resistance Detection:
- Provides susceptibility profiles for informed therapeutic decision-making and antimicrobial stewardship.
- STI Management:
- Identifies common sexually transmitted pathogens for appropriate treatment and prevention.
Advantages
| Feature | Benefit |
|---|---|
| Complete Diagnosis | Combines identification, enumeration, and susceptibility testing in one panel. |
| Time-Saving | Results available within 24–48 hours, reducing diagnostic turnaround time. |
| Ease of Interpretation | Clear colorimetric changes for straightforward results analysis. |
| Automatic Reading Compatibility | Supports automated systems for enhanced efficiency. |
| Auxiliary Products | Simplifies sample transport and testing with complementary products. |
Comparison with Similar Systems
| Feature | Mycoplasma System Plus (Liofilchem) | Mycoplasma IST (BioMérieux) | Generic Culture Methods |
|---|---|---|---|
| Microorganisms Detected | Mycoplasmas, Candida, T. vaginalis | Mycoplasmas | Limited |
| Antimicrobial Susceptibility | Yes | Yes | Requires separate tests |
| Ease of Use | Clear color changes | Requires equipment | Labor-intensive |
| Incubation Time | 24–48 hours | 48–72 hours | 72+ hours |
| Cost Efficiency | High | Moderate | Low |
Regulatory Compliance
- Manufactured under ISO-certified quality systems.
- Complies with CLSI and EUCAST guidelines for susceptibility testing.
The Mycoplasma System Plus is an essential tool for laboratories aiming to streamline workflows and deliver rapid, accurate diagnoses for urogenital infections, providing significant clinical and operational benefits.




 0
0