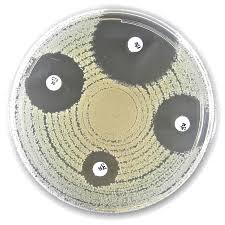
Liofilchem X and V Factor Test discs
Catalog No :
CAS Number :
Brand :
In Stock
used for identification of Haemophilus spp.
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Liofilchem™ X, V, and V+X Factor Test is a diagnostic tool designed for the differentiation of Haemophilus species based on their growth factor requirements. The test uses paper discs impregnated with Hemin (X Factor), Nicotinamide Adenine Dinucleotide (NAD, V Factor), or both (V+X Factor), allowing for the identification of Haemophilus spp. by evaluating growth around the respective discs.
Key Features
- Species Differentiation:
- Identifies Haemophilus species based on their specific requirements for X and V growth factors.
- Ready-to-Use Discs:
- Pre-prepared discs for convenience and reliability.
- Validated for Clinical Relevance:
- Suitable for testing a wide range of Haemophilus spp., including H. influenzae and H. parainfluenzae.
- Standards-Compliant:
- Complies with international diagnostic standards for microbiological testing.
Kit Contents
- 2 cartridges of 50 discs each:
- X Factor Discs (Hemin).
- V Factor Discs (NAD).
- V+X Factor Discs (Hemin + NAD).
Method Principle
- Differentiation Based on Growth Requirements:
- Haemophilus spp. have varying needs for X and V factors, which are present in blood. This test utilizes discs impregnated with these factors to differentiate species by evaluating bacterial growth patterns on blood-free media.
Test Procedure
- Prepare Test Plate:
- Inoculate the entire surface of a blood-free agar plate (e.g., Tryptic Soy Agar, Brain Heart Infusion Agar, or Mueller Hinton Agar) with a pure suspension of the test organism.
- Apply Discs:
- Place one X, one V, and one V+X Factor disc on the agar in an equilateral triangle, ensuring at least 30-35 mm between discs.
- Incubation:
- Incubate at 35 ± 2°C for 24–48 hours in a 5–10% carbon dioxide atmosphere.
- Interpret Results:
- Observe growth around the discs to determine the organism's requirements:
- Growth around X and V+X discs: Requires X factor.
- Growth around V and V+X discs: Requires V factor.
- Growth only around V+X disc: Requires both factors.
- Observe growth around the discs to determine the organism's requirements:
Interpretation Example
| Species | Growth Around Disc | Interpretation |
|---|---|---|
| H. influenzae | - - + | Requires both X and V. |
| H. parainfluenzae | - + + | Requires V only. |
| H. ducreyi | + - + | Requires X only. |
| H. haemolyticus | - - + | Requires both X and V. |
Storage and Shelf Life
- Storage:
- Store at 2–8°C in original packaging, away from heat and direct sunlight.
- Shelf Life:
- Valid for 2 years when stored as recommended.
Quality Control
| Control Strain | Growth Around Disc | Interpretation |
|---|---|---|
| Haemophilus influenzae ATCC® 19418 | - - + | Requires X and V factors. |
| Aggregatibacter aphrophilus ATCC® 7901 | - + + | Requires V factor. |
Advantages
- Accurate Differentiation:
- Provides clear results for species identification based on X and V factor requirements.
- Easy to Use:
- Simple setup with pre-prepared discs reduces preparation time.
- Cost-Effective:
- Allows for simultaneous testing of multiple organisms on a single plate.
- Validated:
- Performance tested with control strains to ensure reliability.
Warnings and Precautions
- For in vitro diagnostic use only.
- Use with pure cultures to ensure accuracy.
- Follow standard aseptic techniques to prevent cross-contamination.
- Dispose of used materials according to local regulations.
Limitations
- Additional Testing Required:
- Growth factor requirements alone are not sufficient for complete species identification; further biochemical tests are recommended.
- Nutrient Carryover:
- Avoid contamination during inoculation as carryover nutrients may affect results.
Packaging
| Product | Quantity | Catalog Number |
|---|---|---|
| X Factor Test | 2 x 50 discs | 9503 |
| V Factor Test | 2 x 50 discs | 9504 |
| V+X Factor Test | 2 x 50 discs | 9505 |
References
- Public Health England. (2019). X and V Factor Test. UK Standards for Microbiology Investigations.
- Kilian M. (1980). Haemophilus differentiation using growth factors. Manual of Clinical Microbiology.
- Hammond GW et al. (1978). Evaluation of hemin and NAD requirements in Haemophilus spp.. J. Clin. Microbiology.
Why Choose Liofilchem X, V, V+X Factor Test?
- Proven Accuracy: Trusted for reliable differentiation of Haemophilus species.
- Ease of Use: Simplifies identification with pre-impregnated discs.
- Validated Performance: Tested with control strains to ensure reliability.
- Type: V Factor F Factor V + F Factor




 0
0