Liofilchem® SensiQuattro Candida, EUCAST
Catalog No :
CAS Number :
Brand :
In Stock
Panel for determining the susceptibility testing of Candida spp. with antimycotic agents.
Specifications:
| Application | Microbial Identification/AST | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Microbial ID System | Forms | Kit with Various components |
| Product Brand | Liofilchem | ||
| Product Grade | Microbiology grade | ||
The SensiQuattro Candida EU is a 32-well diagnostic panel designed for the susceptibility testing of Candida species against 8 different antifungal agents at four serial concentrations (doubling dilutions). By correlating results with EUCAST (European Committee on Antimicrobial Susceptibility Testing) clinical breakpoints, the system allows for precise categorization of Candida spp. as Sensitive (S) or Resistant (R) to antifungal agents.
This panel is an essential tool for clinical laboratories, facilitating accurate antifungal susceptibility profiling for guiding effective antifungal therapies.
Key Features:
- Comprehensive Antifungal Profiling:
- Includes 8 antifungal agents:
- Fluconazole, Voriconazole, Posaconazole, Caspofungin, Micafungin, Anidulafungin, Flucytosine, and Amphotericin B.
- Each agent tested at four concentrations, ensuring precise Minimum Inhibitory Concentration (MIC) determination.
- Includes 8 antifungal agents:
- EUCAST-Validated Results:
- Results are interpretable using EUCAST clinical breakpoints, ensuring compatibility with internationally recognized standards for antifungal susceptibility testing.
- Convenient 32-Well Panel Design:
- Includes a Growth Control well for verifying the vitality of the Candida culture.
- Rapid and Reliable Testing:
- Results available within 24 ± 2 hours of incubation.
- Quality Assurance:
- Each batch undergoes rigorous quality control using reference Candida strains, including:
- Candida krusei (ATCC 6258)
- Candida parapsilosis (ATCC 22019)
- Candida albicans (ATCC 10231 and ATCC 90028)
- Candida tropicalis (ATCC 750).
- Each batch undergoes rigorous quality control using reference Candida strains, including:
Applications:
- Antifungal Susceptibility Testing (AST):
- Determine the susceptibility of Candida species to clinically relevant antifungals.
- Aid in therapeutic decisions for treating Candida infections.
- Clinical and Research Laboratories:
- Used in both diagnostic and research settings for studying antifungal resistance trends.
Kit Contents:
- SensiQuattro Candida EU Panels:
- Ref. 76033: 20 panels (32 wells each).
- Ref. 79033: 4 panels (32 wells each).
- 20 or 4 Tubes of Physiological Solution (6 mL each).
- Instruction Sheet and Results Data Form.
Additional Requirements (Not Included in Kit):
- Multichannel pipette (50-300 µL).
- Tips for multichannel pipette (1000 tips).
- Solution reservoir for multichannel pipetting.
- McFarland 0.5 standard for inoculum preparation.
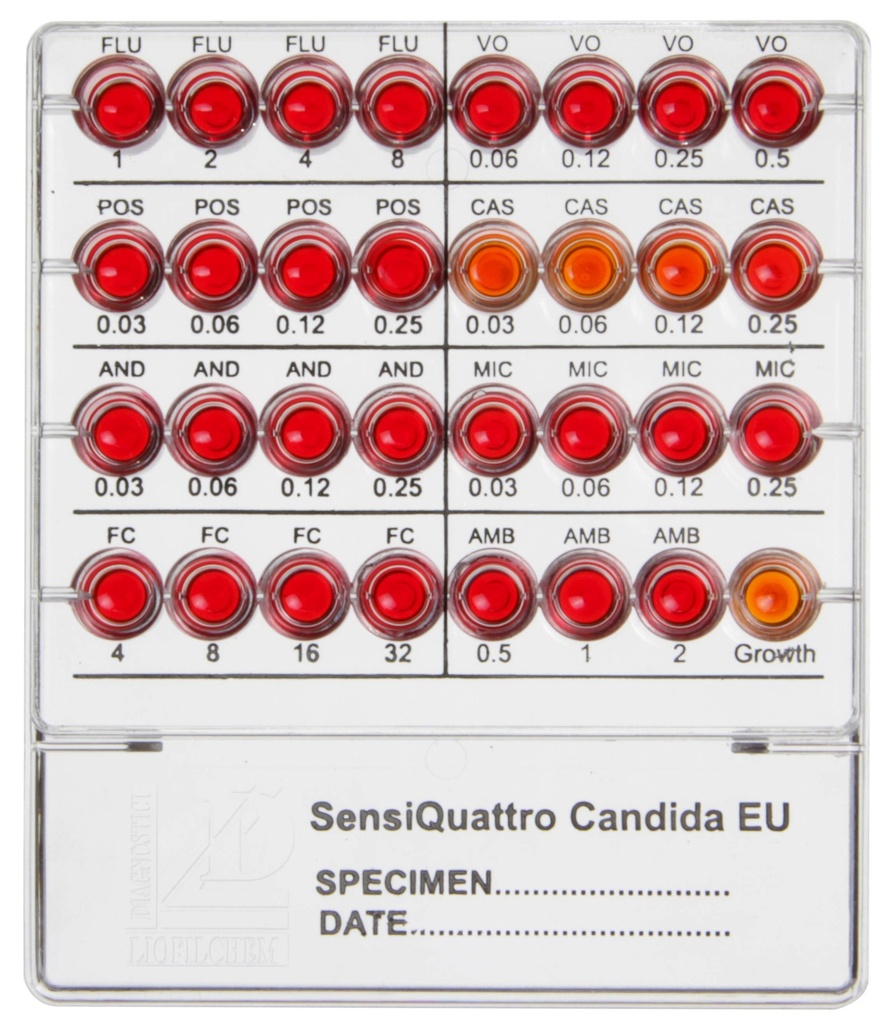
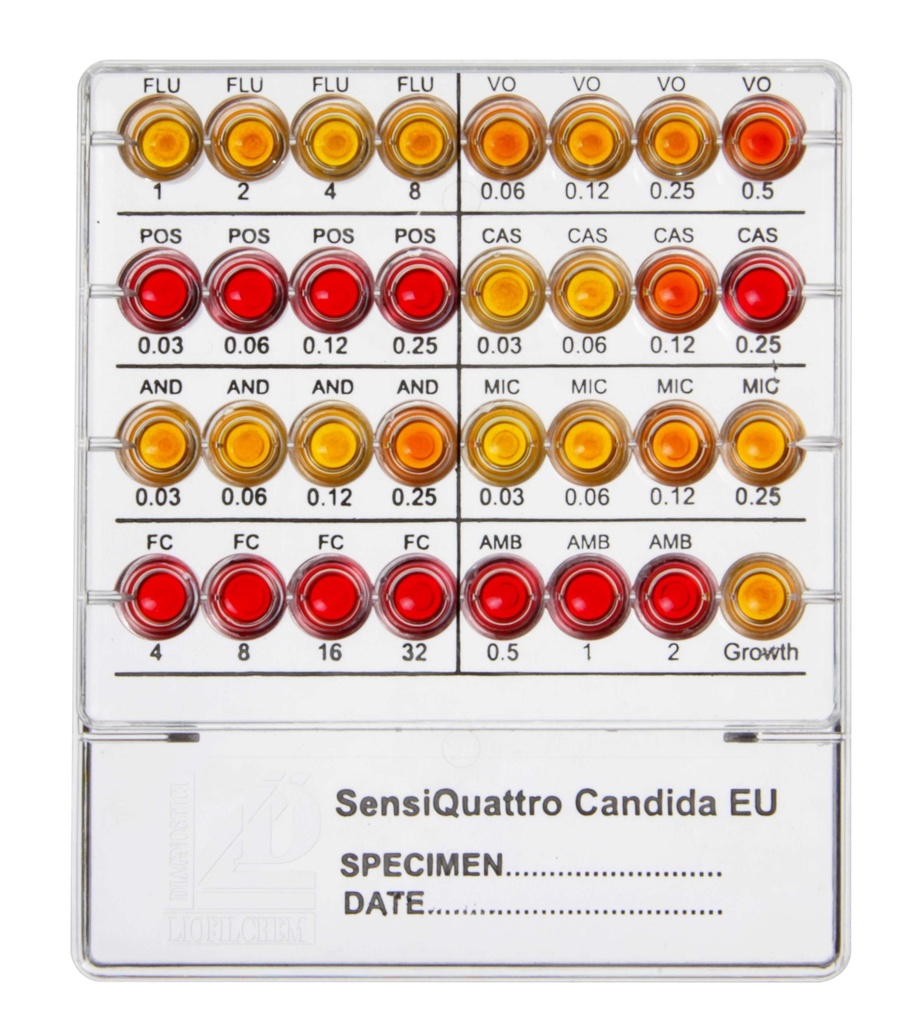
Well Configuration and Antimycotic Agents:
| Well | Antimycotic | Concentration (µg/mL) |
|---|---|---|
| FLU | Fluconazole | 1, 2, 4, 8 |
| VO | Voriconazole | 0.06, 0.12, 0.25, 0.5 |
| POS | Posaconazole | 0.03, 0.06, 0.12, 0.25 |
| CAS | Caspofungin | 0.03, 0.06, 0.12, 0.25 |
| MIC | Micafungin | 0.03, 0.06, 0.12, 0.25 |
| AND | Anidulafungin | 0.015, 0.03, 0.06, 0.12 |
| FC | Flucytosine | 4, 8, 16, 32 |
| AMB | Amphotericin B | 0.5, 1, 2 |
| Growth | Growth control well | N/A |
Principle of the Method:
- Standardized Inoculum:
- Colonies of Candida spp. are suspended in the provided Physiological Solution to match the opacity of a McFarland 0.5 standard.
- Panel Inoculation:
- Using a multichannel pipette, 0.15 mL of inoculum is added to each well.
- The panel is then covered and incubated at 34.5–35.5°C for 24 ± 2 hours.
- Results Interpretation:
- Red color indicates microbial growth inhibition (antimycotic is effective).
- Yellow/Orange color indicates microbial growth (antimycotic is ineffective).
- Results are compared to EUCAST breakpoints for interpretation as Sensitive (S) or Resistant (R).
Interpretation of Results:
| Well Color | Microbial Growth |
|---|---|
| Red | Growth Inhibited |
| Yellow/Orange | Growth Present |
Quality Control and Validation:
- Rigorous Quality Control:
- Each batch is tested using well-documented Candida strains (e.g., C. albicans, C. parapsilosis, C. krusei).
- EUCAST-Compliant Validation:
- Results aligned with EUCAST breakpoints for clinical accuracy and standardization.
Storage and Stability:
- Storage:
- Store at 2–8°C, away from light, in the original packaging.
- Product remains stable during transport for up to 4 days at 18–25°C or 48 hours at 35–39°C.
- Shelf Life:
- Valid until the expiry date indicated on the label. Discard if visible deterioration occurs.
Disposal of Used Material:
- All used panels and materials that come into contact with the sample must be decontaminated and disposed of following standard laboratory guidelines for handling potentially infectious material.
Advantages:
- Comprehensive Antifungal Testing:
- Covers a wide range of antifungal agents at multiple concentrations.
- Standardized Protocol:
- EUCAST breakpoints ensure high clinical relevance.
- Ease of Use:
- Pre-prepared, desiccated antimycotic wells simplify workflow.
- Fast Results:
- Obtain susceptibility profiles within 24 ± 2 hours.
- Reliable and Validated:
- Rigorous quality control and validation with reference strains.
Packaging Information:
| Product | Reference | Contents |
|---|---|---|
| SensiQuattro Candida EU | 76033 | 20 panels |
| SensiQuattro Candida EU | 79033 | 4 panels |
Regulatory and Safety Information:
- Intended for in vitro diagnostic use only.
- Use by appropriately trained personnel in a professional laboratory setting, adhering to aseptic techniques.
- Pack Size: for 20 Tests for 4 Tests




 0
0