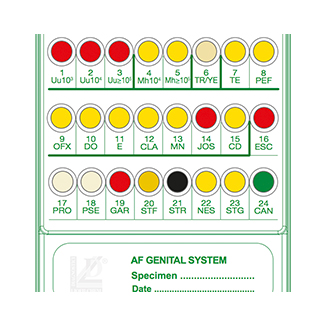
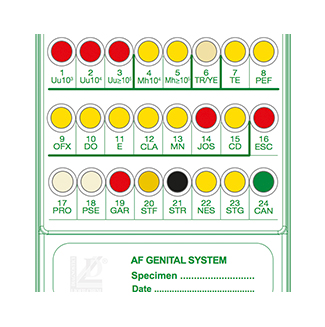
Liofilchem® A.F. Genital System for 20 Tests
Catalog No :
CAS Number :
Brand :
In Stock
A 24-well panel containing biochemical substrata and antimicrobial drugs for detection, presumptive identification, and susceptibility testing of microorganisms from urogenital specimens (vaginal swab, urethral swab, seminal fluid, urine).
Specifications:
| Application | Clinical microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Microbial ID System |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The A.F. Genital System is an innovative 24-well panel developed by Liofilchem® for the detection, presumptive identification, and susceptibility testing of microorganisms associated with urogenital infections. This system streamlines microbiological workflows by combining screening, identification, and susceptibility testing into a single, compact panel.
 Sensitivity
Sensitivity Resistance
Resistance
Key Features and Benefits
- Comprehensive Testing in One Panel:
- Combines four types of microbiological testing:
- Enumeration of urogenital mycoplasma (Mycoplasma hominis and Ureaplasma spp.).
- Identification of Mycoplasma and Ureaplasma.
- Antimicrobial susceptibility testing.
- Bacterial and fungal screening.
- Combines four types of microbiological testing:
- Ease of Use:
- Simplified test process requiring no complex laboratory equipment.
- Results are read via colorimetric changes in wells for easy interpretation.
- Cost and Time Efficiency:
- Consolidates multiple tests into one, reducing labor, consumables, and time.
- Allows direct biochemical, immunoserological, or microscopic confirmation from culture-positive wells.
- Simultaneous Screening:
- Detects several microorganisms in urogenital specimens like vaginal swabs, urethral swabs, seminal fluid, and urine.
Test Procedure
- Sample Preparation:
- Prepare a clinical specimen suspension from the urogenital sample.
- Inoculation:
- Inoculate the A.F. Genital System panel with the prepared specimen.
- Incubation:
- Incubate at 36 ± 1°C for 24 hours.
- Interpretation:
- Enumeration and Identification:
- Color change in wells indicates growth.
- Enumerate Ureaplasma spp. (Uu) from 10³ to ≥10⁵ CFU/mL.
- Enumerate Mycoplasma hominis (Mh) from 10⁴ to ≥10⁵ CFU/mL.
- Susceptibility Testing:
- Growth indicated by yellow to red color change in wells 7–15 for sensitivity and resistance.
- Bacterial and Fungal Screening:
- Interpret clear color changes for common pathogens such as:
- Escherichia coli, Proteus spp., Pseudomonas spp.
- Gardnerella vaginalis, Candida spp., Streptococcus agalactiae.
- Interpret clear color changes for common pathogens such as:
- Enumeration and Identification:
Clinical Significance
- Urogenital Mycoplasma Testing:
- Detects Mycoplasma hominis and Ureaplasma spp., pathogens associated with pelvic inflammatory disease, bacterial vaginosis, and infertility.
- Bacterial and Fungal Pathogens:
- Screens for significant pathogens causing urogenital infections:
- Escherichia coli (UTI-associated).
- Candida spp. (vaginal yeast infections).
- Neisseria gonorrhoeae (gonorrhea).
- Staphylococcus aureus and Enterococcus faecalis (complicated infections).
- Screens for significant pathogens causing urogenital infections:
- Antimicrobial Stewardship:
- Provides susceptibility profiles to guide targeted therapy and combat antimicrobial resistance.
Regulatory and Compliance Data
- Designed and manufactured under ISO-certified quality systems.
- Compliant with guidelines for clinical microbiology and antimicrobial susceptibility testing as outlined by CLSI and EUCAST.
- Utilized in diagnostic workflows for adherence to international standards for pathogen detection and reporting.
Comparison with Other Systems
| Feature | A.F. Genital System (Liofilchem) | Mycoplasma IST (BioMérieux) | Other Generic Panels |
|---|---|---|---|
| Microorganisms Tested | Mycoplasma, Ureaplasma, bacteria, fungi | Mycoplasma, Ureaplasma | Limited coverage |
| Simultaneous Susceptibility | Yes | Yes | No |
| Ease of Use | Compact, colorimetric results | Requires equipment | Requires manual interpretation |
| Biochemical Confirmation | Direct from well culture broth | Not applicable | Requires separate procedures |
| Cost Efficiency | High | Moderate | Low |
Advantages of the A.F. Genital System
- Broad Microbial Screening:
- Covers multiple pathogens in urogenital specimens, reducing the need for separate tests.
- Direct Workflow Integration:
- Facilitates downstream testing (e.g., biochemical or serological confirmation) directly from culture-positive wells.
- Compact and Cost-Effective:
- Reduces laboratory costs and consumables while improving throughput.
- Comprehensive Susceptibility Testing:
- Simultaneously provides MIC values and resistance patterns for mycoplasmas.
References
- Liofilchem Product Specifications: A.F. Genital System (Ref. 74156).
- Clinical and Laboratory Standards Institute (CLSI) guidelines for urogenital pathogen identification and antimicrobial testing.
- Peer-reviewed studies on the diagnostic significance of urogenital mycoplasmas and associated pathogens.
The A.F. Genital System is an ideal solution for clinical microbiology laboratories looking to streamline workflows while achieving accurate and comprehensive results in urogenital infection diagnostics.
Product Specification not found.
Accessory Products
Add a Review
Customer Review
Richard Atule - bought Liofilchem® A.F. Genital System for 20 Tests




 0
0