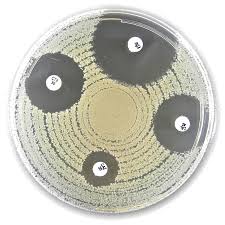
Liofilchem ESBL+AMPC screening disc kit 50 tests
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Clinical microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Liofilchem™ ESBL+AmpC Screen Disc Kit is a diagnostic tool designed for the screening of ESBL (Extended-Spectrum Beta-Lactamase) and AmpC beta-lactamase-producing organisms. These resistance mechanisms are commonly found in Enterobacteriaceae, particularly Escherichia coli and Klebsiella pneumoniae. This kit utilizes Combination Disc Tests (CDT) to differentiate between ESBL, AmpC, and non-beta-lactamase-producing organisms.
Key Features
- Dual Detection:
- Differentiates ESBL and AmpC beta-lactamase production in a single assay.
- Validated for Clinically Relevant Strains:
- Includes Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae.
- CLSI and EUCAST Compliant:
- Follows international guidelines for resistance mechanism detection.
- Convenient Packaging:
- Contains 4 x 50 discs, each packed in blisters with desiccants for optimal storage.
Kit Contents
- 4 x 50 disc cartridges:
- CTX 30 μg: Cefotaxime.
- CTL 30+10 μg: Cefotaxime + Clavulanic Acid.
- CTC 30 μg: Cefotaxime + Cloxacillin.
- CTLC 30+10 μg: Cefotaxime + Clavulanic Acid + Cloxacillin.
Method Principle
- Combination Disc Tests (CDT):
- The test evaluates the inhibition zone sizes around Cefotaxime discs alone and in combination with inhibitors:
- Clavulanic Acid: Inhibits ESBL beta-lactamases.
- Cloxacillin: Inhibits AmpC beta-lactamases.
- Inhibition zones are compared to detect and differentiate ESBL and AmpC production.
- The test evaluates the inhibition zone sizes around Cefotaxime discs alone and in combination with inhibitors:
Test Procedure
- Prepare Test Organism:
- Use a pure, fresh culture to prepare a suspension equivalent to 0.5 McFarland Standard.
- Plate Preparation:
- Spread the bacterial suspension evenly over a Mueller Hinton II Agar plate using a sterile swab.
- Disc Application:
- Apply the discs (CTX, CTL, CTC, and CTLC) onto the plate, ensuring sufficient spacing for measurement.
- Incubation:
- Incubate the plate at 36 ± 1°C for 18–24 hours.
- Measure and Interpret Results:
- Measure inhibition zones and interpret based on the provided table.
Interpretation of Results
| Antibiotic Disc | Difference in Zone Diameters | Interpretation |
|---|---|---|
| Cefotaxime + Clavulanic Acid (CTL) | ≥ 5 mm larger than Cefotaxime (CTX) | ESBL |
| Cefotaxime + Cloxacillin (CTC) | ≥ 5 mm larger than Cefotaxime (CTX) | AmpC |
| Cefotaxime + Clavulanic Acid + Cloxacillin (CTLC) | ≥ 5 mm larger than Cloxacillin (CTC) | ESBL+AmpC |
| All Zones Within 2 mm | No difference | Negative for ESBL/AmpC |
Quality Control
| Control Strain | ESBL Phenotype | AmpC Phenotype |
|---|---|---|
| Klebsiella pneumoniae ATCC® 700603 | Positive | Negative |
| Enterobacter cloacae ATCC® BAA-1143 | Negative | Positive |
| Escherichia coli ATCC® 25922 | Negative | Negative |
Storage and Shelf Life
- Storage:
- Unopened blisters: -20°C to +8°C.
- Opened cartridges: 2-8°C, for up to 7 days.
- Shelf Life: Valid until the expiry date indicated on the label.
Advantages
- Dual Screening: Simultaneously detects ESBL and AmpC production.
- Standardized: Compliant with CLSI and EUCAST guidelines for antimicrobial resistance detection.
- User-Friendly: Pre-packaged discs simplify laboratory workflows.
- Accurate Results: Reliable differentiation between resistance mechanisms.
Warnings and Precautions
- For in vitro diagnostic use (IVD) only.
- Handle all microbiological materials as potentially infectious.
- Follow standard laboratory safety and aseptic techniques.
- Dispose of expired discs and contaminated materials properly.
Packaging
| Description | Quantity | Catalog Number |
|---|---|---|
| Cefotaxime 30 μg (CTX) | 50 discs | 99008 |
| Cefotaxime + Clavulanic Acid (CTL) | 50 discs | 99008 |
| Cefotaxime + Cloxacillin (CTC) | 50 discs | 99008 |
| Cefotaxime + Clavulanic Acid + Cloxacillin (CTLC) | 50 discs | 99008 |
References
- Jacoby G.A. AmpC β-Lactamases. Clin Microbiol Rev. 2009; 22(1): 161–182.
- EUCAST guidelines for resistance mechanism detection. Version 1.0, 2013.
Why Choose Liofilchem ESBL+AmpC Screen Disc Kit?
- Reliable Detection: Differentiates between ESBL and AmpC-producing organisms.
- Standardized Testing: Compliant with international guidelines for microbiological testing.
- Easy-to-Use Format: Pre-packaged discs simplify testing workflows in clinical laboratories.




 0
0