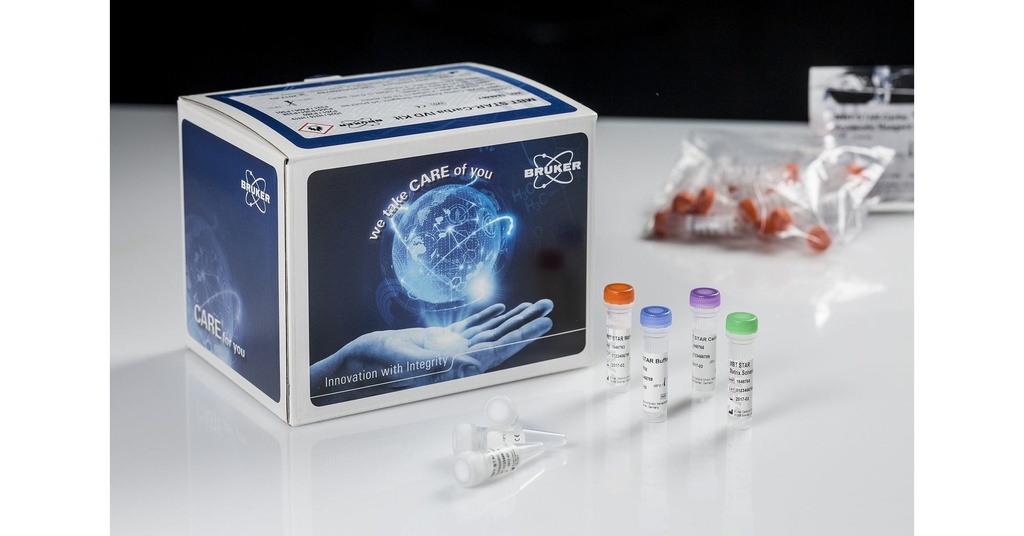
MALDI Biotyper® MBT Sepsityper® IVD Kit, for 50 Tests
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | MALDI-MS |
| Storage Temperature | 2-8°C |
| Product Type | Diagnostic kits |
| Product Brand | Bruker |
| Product Grade | Microbiology grade |
The Bruker MALDI Biotyper® MBT Sepsityper® IVD Kit is designed for the rapid identification of microorganisms directly from positive blood culture bottles. Utilizing Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS), this kit streamlines the diagnostic process, enabling timely and accurate identification of pathogens.
Key Features:
- Rapid Identification: The MBT Sepsityper® IVD Kit facilitates the identification of pathogens within 15 to 20 minutes from positive blood cultures, significantly reducing the time-to-result compared to traditional methods.
- Comprehensive Coverage: The kit's reference library encompasses over 425 different gram-negative and gram-positive bacterial species and groups, as well as yeasts, including emerging pathogens like Candida auris.
- Cost-Effectiveness: By providing a broad range of identifications at a fraction of the cost of expensive syndromic panels with limited species coverage, the MBT Sepsityper® IVD Kit offers an affordable solution for rapid sepsis identification.
Use Cases and Applications:
- Sepsis Management:
- Timely Pathogen Identification: In cases of suspected sepsis, the kit enables rapid identification of the causative microorganisms directly from positive blood cultures. This prompt identification assists clinicians in initiating appropriate antimicrobial therapy sooner, which is crucial for patient outcomes.
- Antimicrobial Stewardship: By accurately identifying pathogens, healthcare providers can tailor antibiotic treatments to the specific organism, reducing the use of broad-spectrum antibiotics and combating antibiotic resistance.
- Clinical Microbiology Laboratories:
- Workflow Efficiency: The MBT Sepsityper® IVD Kit streamlines laboratory workflows by eliminating the need for subculturing and additional biochemical tests, thereby reducing labor and material costs.
- Enhanced Diagnostic Capabilities: With the ability to identify a wide array of microorganisms rapidly, laboratories can provide more comprehensive diagnostic information to clinicians, improving patient management.
- Hospital Infection Control:
- Outbreak Management: Rapid identification of pathogens during infection outbreaks allows for swift implementation of control measures, minimizing the spread within healthcare settings.
- Surveillance Programs: The kit aids in monitoring and tracking infection trends, contributing to effective infection prevention strategies.
Workflow Overview:
Upon a positive blood culture alert, the MBT Sepsityper® IVD Kit is used to process the sample, isolating and purifying microbial cells. These are then analyzed using the MALDI Biotyper® system, which matches the microbial protein profile against its extensive reference library to identify the organism. This entire process typically takes less than 30 minutes, significantly reducing the time compared to traditional culture methods.
In summary, the Bruker MALDI Biotyper® MBT Sepsityper® IVD Kit enhances the speed and accuracy of pathogen identification directly from positive blood cultures. Its application in clinical settings supports timely and appropriate therapeutic interventions, thereby improving patient outcomes and contributing to effective antimicrobial stewardship.




 0
0