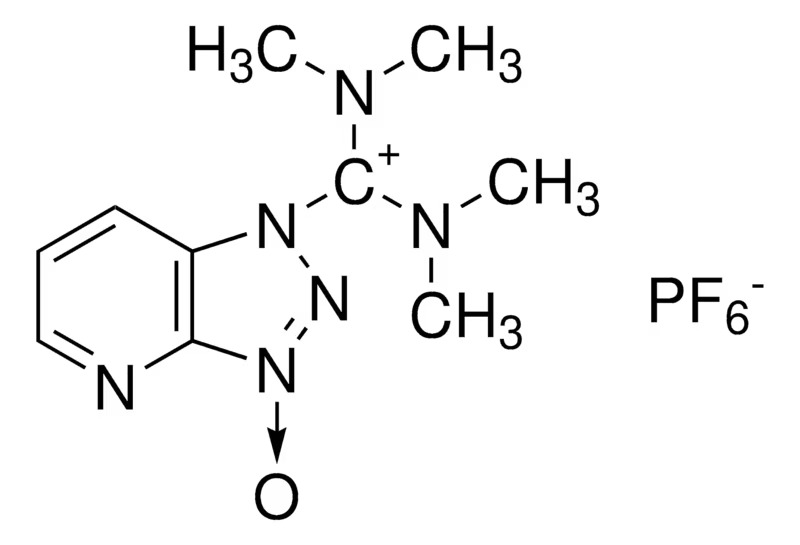
Sigma-Aldrich™ HATU 97%, for peptide synthesis
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Peptide Synthesis | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Amino Acid & Biochemicals | Forms | Solid |
| Product Brand | Sigma-Aldrich | ||
| Product Grade | Analytical grade | Formula | C₁₀H₁₅F₆N₆OP |
HATU (O-(7-Azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate) is a highly efficient amide bond-forming reagent commonly used in solid-phase peptide synthesis (SPPS), pharmaceutical research, and organic chemistry. This reagent:
- Activates carboxyl groups efficiently, leading to rapid and high-yielding peptide bond formation.
- Minimizes racemization, making it an excellent alternative to HBTU and other uronium-based coupling reagents.
- Provides high coupling efficiency in the synthesis of difficult peptide sequences, including sterically hindered and β-sheet-prone peptides.
- Enhances regioselectivity in amide bond formation, allowing for cleaner reactions and higher product purity.
HATU is widely used in peptide synthesis, amide coupling reactions, and bioconjugation strategies, making it a preferred reagent in academic and industrial peptide production.
Key Features & Benefits
✔ High Coupling Efficiency – Forms high-yielding amide bonds even in challenging sequences.
✔ Minimizes Racemization – Reduces epimerization compared to HBTU, DIC, and PyBOP.
✔ Compatible with SPPS & Solution-Phase Synthesis – Suitable for both automated and manual peptide synthesis methods.
✔ Fast Reaction Kinetics – Enhances amide bond formation rates while maintaining high selectivity.
✔ Stable & Versatile – Can be used in carbodiimide-free coupling methods, preventing side reactions.
✔ Well-Characterized & Reliable – Verified via HPLC, melting point analysis, and purity assays.
Chemical Properties & Identifiers
| Property | Value |
|---|---|
| Product Name | HATU, 97% |
| CAS Number | 148893-10-1 |
| Empirical Formula | C₁₀H₁₅F₆N₆OP |
| Molecular Weight | 380.23 g/mol |
| MDL Number | MFCD27957364 |
| UNSPSC Code | 12352302 |
| Optical Activity | Not Specified |
| Form | Solid |
| Melting Point | 183-185°C |
| Assay (HPLC, Verified by HPLC) | ≥97.0% |
| Storage Temperature | 2-8°C |
| Functional Groups | Uronium, Fluorophosphate |
Applications
🔹 Peptide Synthesis & Proteomics Research – Used in Fmoc-based SPPS for high-efficiency amide bond formation.
🔹 Pharmaceutical & Drug Discovery – Essential for peptide drug synthesis and modification.
🔹 Organic Synthesis & Bioconjugation – Enhances amide bond selectivity and functionalization.
🔹 Selective Acylation & Cyclization Reactions – Used in macrocycle formation and bioactive molecule design.
✔ Reported Applications Include:
1️⃣ Aurora A Kinase Inhibitor Synthesis – Applied in targeted drug development for cancer research.
2️⃣ HPLC Enantiomeric Analysis – Used to determine D- and L- acid enantiomers in human plasma.
3️⃣ Peptide Coupling & Solid-Phase Synthesis – Facilitates high-yield amide bond formation in difficult sequences.
4️⃣ N-Arylsulfonamide Peptide Synthesis – Applied in solid-phase approaches to novel peptide-drug conjugates.
Reaction Mechanism & Workflow
HATU-Catalyzed Peptide Coupling Mechanism
1️⃣ Carboxyl Activation – HATU reacts with carboxylic acids (-COOH) in the presence of a base (DIPEA or NMM).
2️⃣ Activated Onium Intermediate Formation – Forms an electron-rich activated ester that enhances amide bond formation.
3️⃣ Nucleophilic Attack – The amine (-NH₂) nucleophile attacks the activated carboxyl group, forming the desired amide bond.
4️⃣ Byproduct Removal – HATU decomposes into a non-reactive hydroxytriazole derivative, minimizing racemization.
✔ Recommended Reaction Conditions:
- Solvent: DMF, DCM, or NMP
- Base: DIPEA (Diisopropylethylamine) or NMM (N-Methylmorpholine)
- Reaction Temperature: Room temperature (20-25°C)
Storage & Handling
📦 Storage Conditions:
- Store at 2-8°C in a dry, tightly sealed container.
- Protect from moisture and prolonged air exposure.
- Dissolve in anhydrous DMF or DCM before use.
⚠ Handling Precautions:
- Use in a fume hood to prevent inhalation of dust.
- Wear gloves, lab coat, and safety goggles while handling.
- Dispose of waste properly according to laboratory regulations.
General References & Research Citations
1️⃣ HATU in Peptide Synthesis & Coupling Strategies – Org. Biomol. Chem., 2021, 19(6), 1573-1581.
2️⃣ Comparison of Peptide Coupling Reagents (HATU vs. HBTU) – J. Pept. Sci., 2019, 25(3), 224-232.
3️⃣ HATU in Drug Development for Kinase Inhibition – J. Med. Chem., 2020, 63(14), 7490-7503.
Regulatory & Safety Information
⚠ For Research Use Only (RUO) – Not intended for diagnostic or therapeutic applications.
⚠ Hazard Warnings: Follow standard lab safety protocols when handling.
Ordering Information
| Catalog Number | Quantity |
|---|---|
| 445460-1G | 1 g |
| 445460-5G | 5 g |
| 445460-25G | 25 g |
| 445460-100G | 100 g |
📦 Shipping Conditions: Ambient
Sigma-Aldrich™ HATU (≥97%) is a highly efficient peptide coupling reagent, ideal for amide bond formation, solid-phase peptide synthesis (SPPS), and organic synthesis. With low racemization, high stability, and rapid reaction kinetics, it is a preferred choice in pharmaceutical research, peptide therapeutics, and bioconjugation strategies.
- Pack Size: 100g 1g 5g 25g




 0
0