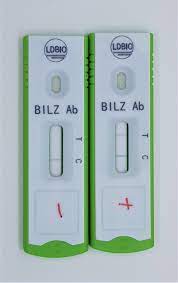
Schisto POC-CCA® Bilharzia (Schistosomiasis) Rapid Test kit
Catalog No :
CAS Number :
Brand :
In Stock
Sample type: Urine
Specifications:
| Application | Diagnostic Testing | ||
| Storage Temperature | Room Temperature | ||
| Product Type | Test Kit | Forms | Kit with Various components |
| Product Brand | ICT | ||
| Product Grade | Medical grade | ||
The Urine Circulating Cathodic Antigen (CCA) Cassette Test is a rapid immunochromatographic assay designed for the qualitative presumptive detection of active Schistosoma infections, particularly Schistosoma mansoni. It may also detect other species such as S. haematobium and S. japonicum in moderate to high-intensity infections.
The test detects CCA antigens excreted in urine by live adult schistosomes, offering a non-invasive, fast, and easy-to-use alternative to stool or urine microscopy. It is particularly suitable for field surveys, endemic population screening, and treatment monitoring.
Key Features and Benefits
- Rapid Results – Provides results within 20 minutes
- Non-Invasive Sampling – Uses midstream urine, no blood or stool handling
- Field-Ready – No equipment required; ideal for use in remote or resource-limited settings
- Post-Treatment Monitoring – CCA levels typically drop within 2–3 weeks of effective treatment
- Stable Storage – Can be stored between 4–40°C without refrigeration
- Clinically Useful – Supports diagnosis in symptomatic individuals when combined with microscopy or serology
Kit Components
- 25 individually packaged CCA test cassettes
- 25 disposable plastic pipettes
- 1 instruction manual
Test Principle
The test utilizes monoclonal antibodies to detect CCA antigens in urine. If antigens are present, a pink test line appears alongside the control line. If no antigens are detected, only the control line appears. A missing control line indicates an invalid test.
Test Procedure
- Collect a midstream urine sample.
- Use the pipette to add 2 drops (100 µL) of urine to the test cassette well.
- Wait 20 minutes.
- Interpret results. Do not interpret after 25 minutes.
Interpretation of Results
| Result | Interpretation |
|---|---|
| Positive | Control and test lines visible – CCA detected |
| Negative | Control line only – No CCA detected |
| Invalid | No control line – Repeat the test |
Note: Haematuria or urinary tract infections may lead to false positives.
Performance Characteristics
| Parameter | Details |
|---|---|
| Sensitivity (High Load) | 100% (≥400 eggs per gram) |
| Sensitivity (Low Load) | ~70% |
| Specificity | ~95% in negative endemic populations |
| Detection Threshold | ~50 worms (based on animal models) |
| Time to Result | 20 minutes |
| Storage Temp. | 4°C – 40°C |
| Sample Type | Midstream urine |
| Shelf Life | Until printed expiration date |
Storage & Sample Handling
- Kit Storage: 4°C to 40°C (do not freeze)
- Urine Samples: Store at 4°C for up to 7 days, or at –20°C for up to 1 year
Limitations
- May be negative in early infections (first 4–8 weeks)
- Not a definitive diagnostic tool; use with clinical history and microscopy
- False positives may occur in cases of haematuria or urinary tract infections
- Cannot differentiate between Schistosoma species
- Test sensitivity varies based on worm burden and infection stage
Applications
- Community surveillance in endemic areas
- School-based screening programs
- Monitoring response to praziquantel treatment
- Supplementing clinical diagnosis in symptomatic patients
- Public health campaigns targeting schistosomiasis control
The Urine CCA Cassette Test offers a reliable, rapid, and field-friendly solution for the detection of active Schistosoma infections, particularly S. mansoni. Its non-invasive format, combined with easy visual interpretation and minimal equipment requirements, makes it ideal for screening, treatment follow-up, and prevalence mapping in endemic regions. While not a standalone diagnostic, it is a valuable tool when used in combination with clinical and laboratory findings to support effective schistosomiasis management and control programs.




 0
0