Liofilchem® Temocillin TMO Antimicrobial Susceptibility Discs, 30 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Resistance |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Temocillin TMO Antimicrobial Susceptibility Discs, 30 µg, are standardized diagnostic tools used for in vitro antimicrobial susceptibility testing (AST) of Enterobacterales, including multidrug-resistant (MDR) strains, against temocillin. These discs are widely employed in clinical microbiology laboratories to determine the effectiveness of temocillin in inhibiting bacterial growth, particularly for beta-lactamase-producing organisms.
Key Features:
- Active Ingredient:
- Temocillin: A beta-lactam antibiotic resistant to most beta-lactamases, including AmpC and extended-spectrum beta-lactamases (ESBLs).
- Ideal for testing Escherichia coli, Klebsiella spp., and other Enterobacterales, particularly in cases of carbapenem resistance.
- Disc Potency:
- Each disc contains 30 µg of temocillin for precise testing.
- Applications:
- Detection of resistance in Enterobacterales.
- Distinguishing between AmpC, ESBL, and carbapenemase-producing strains.
- Supporting targeted antimicrobial therapy for urinary tract infections, bloodstream infections, and nosocomial infections.
- Compliance:
- Follows EUCAST and CLSI guidelines for antimicrobial susceptibility testing.
- Storage Conditions:
- Short-term storage: 2–8°C.
- Long-term storage: -20°C.
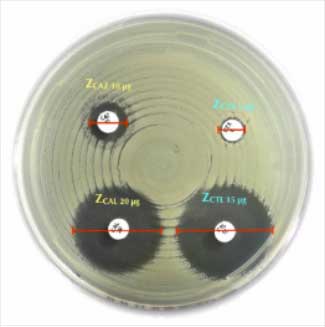
Testing Procedure:
- Preparation:
- Use a fresh culture of the test organism grown on non-selective media.
- Adjust the bacterial suspension to a turbidity equivalent to 0.5 McFarland Standard.
- Inoculation:
- Use a sterile swab to spread the suspension evenly across the surface of Mueller-Hinton Agar (MHA) plates.
- Placement of Discs:
- Place the Liofilchem® Temocillin TMO 30 µg disc onto the agar surface using sterile forceps or a disc dispenser.
- Incubation:
- Incubate the plates at 35 ± 2°C for 16–20 hours in ambient air.
- Interpretation of Results:
- Measure the diameter of the inhibition zone around the disc using a calibrated ruler or automated zone reader.
- Compare the zone diameter to the EUCAST/CLSI breakpoints for temocillin susceptibility.
Quality Control:
| Control Strains | Expected Zone Diameter (mm) | Interpretation |
|---|---|---|
| Escherichia coli ATCC® 25922 | 20–26 | Susceptible |
| Klebsiella pneumoniae ATCC® 700603 | 18–22 | Susceptible |
| Pseudomonas aeruginosa ATCC® 27853 | Resistant | Resistant to Temocillin |
Breakpoints (Based on EUCAST Guidelines):
| Organism | Susceptible (S) Zone Diameter (mm) | Resistant (R) Zone Diameter (mm) |
|---|---|---|
| Enterobacterales | ≥19 | <19 |
Reference Data:
- EUCAST Clinical Breakpoint Tables (v.12.0).
- CLSI M100-S Performance Standards for Antimicrobial Susceptibility Testing.
- Studies have shown temocillin to be effective against ESBL-producing E. coli and Klebsiella pneumoniae, with minimal inhibitory concentrations (MICs) indicating susceptibility.
Comparison with Other Brands:
| Feature | Liofilchem® Temocillin TMO | Competitor A | Competitor B |
|---|---|---|---|
| Disc Potency | 30 µg | 30 µg | 30 µg |
| Storage Conditions | 2–8°C (short-term), -20°C (long-term) | 4–8°C | -20°C |
| EUCAST Compliance | Yes | Yes | No |
| Ease of Use | High | Moderate | High |
| Shelf Life | ≥2 years | ~1 year | 6–12 months |
The Liofilchem® Temocillin TMO 30 µg Antimicrobial Susceptibility Discs provide reliable, accurate, and standardized testing for temocillin susceptibility in clinical microbiology settings. With compliance to EUCAST and CLSI standards, these discs are an essential component in identifying and managing multidrug-resistant Enterobacterales infections.




 0
0