Liofilchem® Ceftazidime+Clavulanic Acid CAL Antimicrobial Susceptibility Discs, 40 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
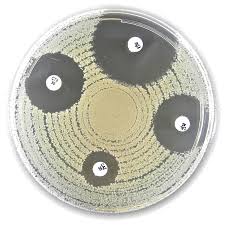
The Liofilchem® Ceftazidime + Clavulanic Acid (CAL) Antimicrobial Susceptibility Discs are designed for the in vitro detection of extended-spectrum β-lactamase (ESBL) production in Gram-negative bacteria, particularly within the Enterobacteriaceae family. Each disc contains a combination of 30 µg of ceftazidime (a third-generation cephalosporin) and 10 µg of clavulanic acid (a β-lactamase inhibitor) to assess bacterial resistance or susceptibility through zone of inhibition testing on agar plates.
These discs are manufactured in compliance with EUCAST and CLSI guidelines and are an essential tool for clinical microbiology laboratories, antimicrobial stewardship programs, and infection control units monitoring ESBL-mediated resistance.
Key Features
- Reliable ESBL Detection: Used in combination with a ceftazidime-only disc for synergy testing to identify ESBL-producing strains.
- Precise Potency: 40 µg total content ensures consistent diffusion and reproducible zone sizes.
- High-Quality Manufacturing: ISO 9001 and ISO 13485 certified production ensures quality and compliance with international standards.
- Wide Compatibility: Suitable for use on Mueller-Hinton agar and with both manual and automated disc dispensers.
- Long Shelf Life: Stable for 2 years under refrigerated conditions (2–8 °C).
Specifications
| Attribute | Details |
|---|---|
| Antibiotic Agent | Ceftazidime + Clavulanic Acid (CAL) |
| Potency | 30 µg Ceftazidime + 10 µg Clavulanic Acid |
| Disc Diameter | 6 mm |
| Test Type | Antimicrobial Susceptibility Testing (AST) |
| Compliance Standards | CLSI, EUCAST |
| Recommended Media | Mueller-Hinton agar |
| Storage Conditions | 2–8 °C |
| Shelf Life | 24 months |
| Packaging | Sealed aluminum blister packs (desiccated) |
Use Cases
1. Detection of ESBL-Producing Enterobacteriaceae
- The disc is used in a synergy test where it is placed near a ceftazidime-only disc. An increase in zone diameter toward the CAL disc indicates ESBL production.
- Commonly used with organisms such as Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and other Enterobacteriaceae.
2. Antimicrobial Surveillance and Resistance Profiling
- Critical for hospital microbiology labs, especially in ICU and infection control settings where tracking multidrug resistance is essential.
- Supports local antibiogram development and informs empirical therapy guidelines.
3. Teaching and Research Applications
- Used in academic and training laboratories to demonstrate resistance mechanisms and validate antimicrobial susceptibility testing methods.
- Suitable for research studies evaluating new β-lactam/β-lactamase inhibitor combinations.
4. Infection Control and Antimicrobial Stewardship
- Identifies patients colonized or infected with ESBL producers, enabling timely isolation procedures.
- Supports evidence-based antibiotic prescribing, minimizing use of carbapenems when possible.
Comparison with Related Liofilchem® AST Discs
| Product | Antibiotic Agent | Primary Use | Special Note |
|---|---|---|---|
| Ceftazidime Disc (30 µg) | Ceftazidime only | General susceptibility testing | Used in synergy with CAL disc |
| Cefotaxime + Clavulanic Acid Disc (30/10 µg) | Cefotaxime + Clavulanic Acid | ESBL detection | Alternate to CAL disc for ESBL ID |
| Piperacillin + Tazobactam Disc (100/10 µg) | Broad-spectrum β-lactam + inhibitor | General susceptibility and synergy | Often used alongside CAL discs |
Unique Advantages of Liofilchem® CAL Discs
- Rapid and Reliable ESBL Identification without molecular diagnostics.
- Excellent Zone Clarity and diffusion for reproducible results.
- Trusted by Clinical Labs Worldwide and manufactured under stringent quality controls.
- Compatible with EUCAST and CLSI interpretation standards for global applicability.
- Cost-effective ESBL screening tool, reducing reliance on expensive molecular tests.
For In Vitro Diagnostic Use Only. Not for therapeutic use.




 0
0