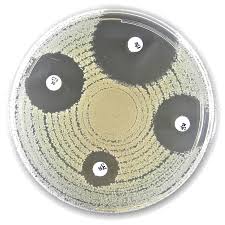
Liofilchem® Gentamicin CN, Antimicrobial Susceptibility Discs, 10 µg (copy)
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Amikacin Antimicrobial Susceptibility Discs, 30 µg, are precision-manufactured antibiotic discs used for determining the susceptibility of microorganisms to amikacin. These discs are used in standardized procedures such as the Kirby-Bauer disk diffusion method for antimicrobial susceptibility testing. The 30 µg amikacin discs are impregnated with a defined quantity of the aminoglycoside antibiotic amikacin, making them ideal for identifying bacterial resistance patterns in clinical and research settings.
Amikacin is a potent broad-spectrum aminoglycoside antibiotic effective against a wide range of Gram-negative and some Gram-positive bacteria. It is commonly used to treat infections caused by multidrug-resistant organisms, particularly in nosocomial settings.
Features:
- Standardized Potency: Each disc contains 30 µg of amikacin, conforming to international standards such as CLSI and EUCAST.
- Precise Diameter: Manufactured to ensure uniform size and antibiotic diffusion for reliable and reproducible results.
- Convenient Packaging: Supplied in cartridges or blister packs, protected from moisture and contamination.
- Quality Assurance: Undergo rigorous quality control testing for potency, diffusion consistency, and sterility.
- Compatibility: Suitable for use with standard agar diffusion methods like Kirby-Bauer and compatible with various susceptibility testing systems.
Composition:
- Active Ingredient: 30 µg amikacin per disc.
- Carrier: High-quality filter paper for uniform diffusion.
- Packaging Material: Moisture-resistant containers with desiccants to ensure product integrity.
Method of Use:
- Preparation:
- Prepare Mueller-Hinton Agar plates according to standardized protocols.
- Allow the medium to solidify and reach room temperature.
- Inoculation:
- Prepare a bacterial suspension equivalent to a 0.5 McFarland standard.
- Evenly inoculate the agar surface using a sterile swab to create a lawn of growth.
- Application of Discs:
- Using a sterile disc dispenser or forceps, place the Amikacin 30 µg discs onto the agar surface.
- Ensure the discs are evenly spaced and at least 24 mm apart from center to center.
- Incubation:
- Invert the plates and incubate aerobically at 35 ± 2°C for 16-18 hours.
- Interpretation:
- Measure the diameter of the zone of inhibition around the disc in millimeters.
- Compare the measurements to interpretive criteria provided by CLSI/EUCAST to classify the isolate as susceptible, intermediate, or resistant.
Quality Control:
Recommended control strains to verify performance:
- Escherichia coli ATCC® 25922: Expected zone range: 19-25 mm.
- Pseudomonas aeruginosa ATCC® 27853: Expected zone range: 19-25 mm.
- Staphylococcus aureus ATCC® 25923: Expected zone range: 19-25 mm.
Storage:
- Store at 2-8°C in the original container with desiccant.
- Protect from moisture, light, and temperature fluctuations.
- Do not use beyond the expiry date indicated on the packaging.
Applications:
- Clinical Microbiology Laboratories:
- Detection of antimicrobial resistance in Gram-negative and some Gram-positive bacteria.
- Pharmaceutical Research:
- Evaluation of antibiotic efficacy against clinical isolates.
- Public Health Monitoring:
- Surveillance of antibiotic resistance trends.
Limitations:
- Results should be interpreted in conjunction with other clinical and microbiological data.
- Not suitable for direct use on non-standardized or non-validated media.
- Disc performance may be affected by improper storage conditions.
Regulatory Compliance:
- Manufactured in accordance with CLSI/EUCAST guidelines.
- CE-marked for in vitro diagnostic use.
Safety Precautions:
- For in vitro diagnostic use only.
- Use by trained laboratory professionals.
- Dispose of used materials according to institutional biosafety protocols.
References:
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.




 0
0