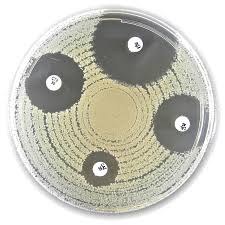
Liofilchem® Cefoperazone (CFP) Antimicrobial Susceptibility Discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Cefoperazone (CFP) Antimicrobial Susceptibility Discs are designed for in vitro testing of bacterial sensitivity to Cefoperazone, a third-generation cephalosporin antibiotic with broad-spectrum activity against Gram-negative and select Gram-positive bacteria.
Each disc contains a defined quantity of Cefoperazone and is manufactured in compliance with EUCAST and CLSI performance standards. Liofilchem discs ensure precision, reproducibility, and stability for accurate measurement of inhibition zones during routine antimicrobial susceptibility testing.
Key Features
- Cefoperazone (CFP): broad-spectrum β-lactam antibiotic effective against Enterobacteriaceae, Pseudomonas spp., Haemophilus spp., and other Gram-negative bacilli.
- Available in two concentrations – 30 µg and 75 µg.
- Manufactured according to CLSI and EUCAST guidelines.
- Each batch validated for potency, uniform diffusion, and stability.
- Long shelf life with desiccated, nitrogen-sealed packaging.
- Compatible with manual or automated Kirby–Bauer disc diffusion systems.
- Suitable for use in clinical, pharmaceutical, and research microbiology laboratories.
Principle of the Method
The disc diffusion (Kirby–Bauer) technique determines the susceptibility of bacterial isolates by measuring the inhibition zone around antibiotic-impregnated discs placed on inoculated agar plates (usually Mueller–Hinton Agar).
The size of the inhibition zone is compared with CLSI or EUCAST reference tables to determine whether the test organism is susceptible, intermediate, or resistant to Cefoperazone.
Product Specifications
| Ref. Code | Description | Antibiotic Content | Packaging | Quantity |
|---|---|---|---|---|
| 9016 | Cefoperazone (CFP) 30 µg | 30 µg | Cartridge | 5 × 50 Discs |
| 9016/2 | Cefoperazone (CFP) 30 µg | 30 µg | Canister | 250 Discs |
| 9108 | Cefoperazone (CFP) 75 µg | 75 µg | Cartridge | 5 × 50 Discs |
| 9108/2 | Cefoperazone (CFP) 75 µg | 75 µg | Canister | 250 Discs |
Storage and Stability
- Store at 2–8 °C in a dry, airtight container.
- Allow discs to reach room temperature before use.
- Protect from humidity and direct sunlight.
- Shelf life: Refer to product label (typically 24–36 months).
Quality Assurance
- Manufactured under ISO 9001 and ISO 13485 certified quality systems.
- Potency and diffusion characteristics validated per CLSI M100 and EUCAST criteria.
- Each lot supplied with a certificate of analysis (CoA) ensuring compliance with regulatory standards.
Safety and Handling
- For in vitro diagnostic use only.
- Use by qualified microbiology personnel under aseptic laboratory conditions.
- Follow biosafety regulations for handling pathogenic microorganisms.
- Dispose of used discs and plates as biohazard waste per institutional protocols.
Ordering Information
| Product | Ref. | Disc Content | Packaging Type | Quantity |
|---|---|---|---|---|
| Cefoperazone CFP 30 µg | 9016 | 30 µg | Cartridge | 5 × 50 Discs |
| Cefoperazone CFP 30 µg | 9016/2 | 30 µg | Canister | 250 Discs |
| Cefoperazone CFP 75 µg | 9108 | 75 µg | Cartridge | 5 × 50 Discs |
| Cefoperazone CFP 75 µg | 9108/2 | 75 µg | Canister | 250 Discs |
Liofilchem® Cefoperazone (CFP) Antimicrobial Susceptibility Discs provide a reliable, standardized tool for determining bacterial resistance patterns using the Kirby–Bauer method. Offering superior precision, batch-to-batch consistency, and global regulatory compliance, these discs are ideal for clinical diagnostics, antibiotic surveillance programs, and research applications.
- package Type: In Cartridge In Canister
- Drug Concentration: 30 µg 75 µg




 0
0