Liofilchem Piperacillin-tazobactam TZP 110 µg, 5x50 Disc
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Liofilchem® Piperacillin-Tazobactam TZP Antimicrobial Susceptibility Discs (available in 36 µg and 110 µg concentrations) are specialized diagnostic tools used in clinical microbiology laboratories for determining the susceptibility of bacterial pathogens to the combination antibiotic piperacillin-tazobactam. This combination is widely utilized to treat a variety of infections caused by Gram-negative and Gram-positive organisms, including multidrug-resistant strains.
Key Features
- Active Ingredient:
- Contains a fixed ratio of piperacillin (a broad-spectrum beta-lactam antibiotic) and tazobactam (a beta-lactamase inhibitor).
- 36 µg discs and 110 µg discs offer flexibility for routine and specialized susceptibility testing.
- Compliance and Standards:
- Manufactured in accordance with Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.
- Ensures reproducible and accurate results for clinical and research purposes.
- High-Quality Construction:
- Uniformly impregnated antimicrobial discs made from high-grade absorbent paper.
- Sterile and individually packaged to maintain potency and precision.
- Storage and Shelf Life:
- Store at 2–8°C to maintain efficacy.
- Typical shelf life of 24 months from the date of manufacture when stored correctly.
- Disc Format:
- Compatible with standard manual or automated disc dispensers.
- Available in packs of 50 or 100 discs.
Applications
- Clinical Microbiology:
- Used to guide therapy by determining the susceptibility of bacterial isolates to piperacillin-tazobactam.
- Effective for Gram-negative bacteria like Pseudomonas aeruginosa and Escherichia coli.
- Antimicrobial Resistance Studies:
- Detects resistance mechanisms, including beta-lactamase production.
- Helps identify multidrug-resistant organisms (MDROs).
- Research and Pharmaceutical Development:
- Used in laboratories for studying the efficacy of piperacillin-tazobactam against various pathogens.
- Facilitates the evaluation of new resistance patterns and drug combinations.
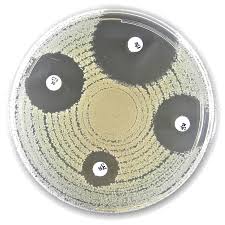
Testing Procedure
- Preparation:
- Prepare a bacterial suspension equivalent to 0.5 McFarland standard.
- Inoculate onto a Mueller-Hinton Agar plate.
- Application:
- Place the 36 µg or 110 µg Piperacillin-Tazobactam disc onto the inoculated plate using sterile forceps or a disc dispenser.
- Incubation:
- Incubate plates at 35°C ± 2°C for 16–18 hours.
- Result Interpretation:
- Measure the diameter of the inhibition zone.
- Compare measurements with CLSI or EUCAST breakpoint tables to determine susceptibility.
Susceptibility Profile
- Bacteria Susceptible to Piperacillin-Tazobactam:
- Pseudomonas aeruginosa
- Escherichia coli
- Klebsiella pneumoniae
- Enterobacter species
- Proteus mirabilis
- Resistance Mechanisms Identified:
- Beta-lactamase production.
- Efflux pump activity and porin loss.
Comparison of 36 µg and 110 µg Discs
| Feature | 36 µg Piperacillin-Tazobactam Disc | 110 µg Piperacillin-Tazobactam Disc |
|---|---|---|
| Primary Use | Routine clinical susceptibility testing | Specialized testing for resistant strains |
| Zone Diameter | Smaller inhibition zones | Larger inhibition zones due to higher drug concentration |
| Organism Focus | Common clinical isolates | Strains with higher resistance profiles |
Quality Control
- Positive Control Strains:
- Escherichia coli ATCC® 25922: Confirms consistent disc potency and proper performance.
- Pseudomonas aeruginosa ATCC® 27853: Ensures the accuracy of results for Gram-negative testing.
- Negative Control Strains:
- Resistant strains produce no inhibition zone, validating the test system.
Advantages
- Reliable Performance:
- Ensures accurate detection of susceptibility and resistance.
- Flexible Testing:
- Dual concentrations (36 µg and 110 µg) allow for routine and advanced diagnostics.
- Global Standards Compliance:
- Conforms to CLSI and EUCAST guidelines for universal applicability.
- High Sensitivity:
- Effective in identifying beta-lactamase-producing and multidrug-resistant organisms.
Conclusion
The Liofilchem® Piperacillin-Tazobactam TZP Antimicrobial Susceptibility Discs, 36 µg and 110 µg, are indispensable in clinical and research laboratories for determining bacterial susceptibility to this critical antibiotic combination. These discs provide high reliability, flexibility, and compliance with international standards, making them essential for combating antimicrobial resistance and guiding effective patient care.
- Drug Concentration: 36 µg 110 µg




 0
0