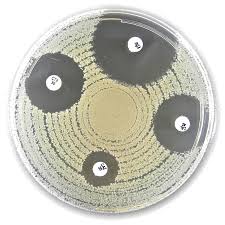
Liofilchem® Meropenem MRP, Antimicrobial Susceptibility Discs, 10 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Reliable Tool for Bacterial Susceptibility Testing in Clinical Microbiology
The Liofilchem® Meropenem MRP Antimicrobial Susceptibility Discs (10 µg) are standardized diagnostic tools used in clinical microbiology laboratories to determine bacterial susceptibility or resistance to meropenem, a carbapenem-class broad-spectrum antibiotic. These discs are a key component of the Kirby-Bauer disk diffusion method, ensuring accurate and reproducible antimicrobial susceptibility testing (AST) to guide appropriate antibiotic therapy.
These discs help identify carbapenem-resistant bacteria (CRB) and carbapenemase-producing organisms (CPOs), which are of significant concern in healthcare-associated infections (HAIs) and multi-drug-resistant (MDR) pathogens.
Key Features & Benefits
✅ Standardized Potency – Each disc contains 10 µg of meropenem, ensuring consistent testing
✅ High-Quality Manufacturing – Produced under strict quality controls for batch-to-batch uniformity
✅ Reliable & Reproducible Results – Designed for use in Kirby-Bauer disk diffusion testing
✅ Carbapenem Resistance Detection – Detects resistant pathogens and carbapenemase-producing organisms (CPOs)
✅ Compliant with International Standards – Meets EUCAST and CLSI antimicrobial susceptibility guidelines
Use Cases
1. Determining Antimicrobial Susceptibility
🔬 Clinical Diagnostics
- Used in clinical microbiology labs to assess bacterial susceptibility to meropenem
- Helps guide appropriate antibiotic therapy for Gram-negative bacterial infections
- Commonly tested against:
- Escherichia coli (E. coli)
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Acinetobacter baumannii
2. Detection of Resistance Mechanisms
⚠️ Carbapenemase Production
- A reduced or absent zone of inhibition around the meropenem disc may indicate the presence of carbapenemase-producing bacteria (CPOs)
- Used in conjunction with modified Hodge test (MHT), Carba NP, or molecular assays to confirm resistance
3. Epidemiological Surveillance
📊 Resistance Monitoring
- Aids public health authorities and infection control teams in tracking meropenem-resistant bacteria
- Supports antimicrobial stewardship programs (ASP) in hospitals, reference labs, and research institutions
Application Procedure: Kirby-Bauer Disk Diffusion Method
1. Sample Preparation
- Prepare a standardized bacterial suspension (0.5 McFarland turbidity)
- Use Mueller-Hinton agar plates as recommended by CLSI & EUCAST
2. Inoculation & Placement
- Swab the bacterial suspension uniformly onto the agar plate
- Place the Meropenem 10 µg disc on the inoculated agar surface
3. Incubation & Measurement
- Incubate at 35 ± 2°C for 16-20 hours
- Measure the zone of inhibition around the disc
- Compare the diameter to EUCAST/CLSI interpretive criteria to classify as:
- Susceptible (S)
- Intermediate (I)
- Resistant (R)
Interpretation Criteria (Example - EUCAST Guidelines)
Note: Values may vary based on the latest EUCAST/CLSI updates.
| Bacterial Pathogen | Susceptible (S) (≥ mm) | Resistant (R) (≤ mm) |
|---|---|---|
| Escherichia coli | ≥ 22 mm | ≤ 21 mm |
| Klebsiella pneumoniae | ≥ 22 mm | ≤ 21 mm |
| Pseudomonas aeruginosa | ≥ 20 mm | ≤ 19 mm |
| Acinetobacter baumannii | ≥ 21 mm | ≤ 20 mm |
Ordering Information
| Product | Packaging |
|---|---|
| Liofilchem® Meropenem MRP 10 µg Discs | 50 Discs per Cartridge, 5 Cartridges per pack |
| Liofilchem® AST Disc Dispenser | Compatible with Liofilchem Disc Cartridges |
Storage & Stability
- Store at 2-8°C (Refrigerated) in sealed packaging
- Protect from moisture, light, and contamination
- Do not use expired or compromised discs
Regulatory & Safety Information
⚠️ For In Vitro Diagnostic (IVD) Use Only – Not intended for direct patient treatment
⚠️ CE-Marked & CLSI/EUCAST Compliant – Ensures quality and regulatory adherence
- Biosafety Precautions: Handle bacterial cultures under Biosafety Level (BSL-2) or higher conditions
- Waste Disposal: Follow local regulations for disposal of biohazardous materials
Why Choose Liofilchem® Meropenem MRP Susceptibility Discs?
🔹 Accurate & Reliable AST Testing – Ensures precise antimicrobial susceptibility results
🔹 Detects Carbapenem-Resistant Bacteria – Essential for infection control & surveillance
🔹 Compliant with Global Standards – Meets CLSI, EUCAST, and ISO guidelines
🔹 Compatible with Kirby-Bauer Testing – Easy integration into microbiology workflows
🔹 Proven Performance in Clinical & Research Labs – Used in hospitals, reference labs, and universities
For bulk orders, technical support, or additional information, contact LabMart Limited today.




 0
0