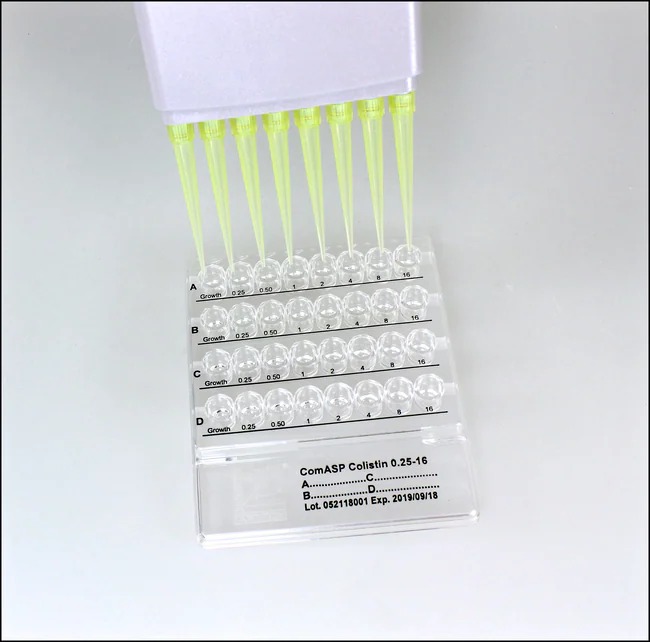
Liofilchem® ComASP™ Colistin / Piperacillin-tazobactam, 4 Tests
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Clinical microbiology |
| Storage Temperature | -20°C |
| Product Type | Microdilution Panel |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® ComASP™ Colistin / Piperacillin-tazobactam MIC Test (4 tests) is designed for the detection of antimicrobial resistance in Gram-negative bacteria, specifically targeting resistance to Colistin and Piperacillin-tazobactam. This MIC (Minimum Inhibitory Concentration) test is used in clinical microbiology labs for identifying the susceptibility or resistance profile of clinically important pathogens such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae.
Colistin is a polymyxin antibiotic used as a last-resort treatment for infections caused by multidrug-resistant (MDR) Gram-negative bacteria. On the other hand, Piperacillin-tazobactam is a broad-spectrum beta-lactam/beta-lactamase inhibitor combination that is commonly used against Enterobacteriaceae, Pseudomonas, and other Gram-negative infections.
This dual testing combination helps identify bacterial strains with resistance to both these important antibiotics and assists in guiding treatment decisions for MDR infections.
Test Description and Mechanism:
- Product Name: Liofilchem® ComASP™ Colistin / Piperacillin-tazobactam MIC Test (4 Tests)
- Form: MIC test strips (single strips for each antibiotic combination)
- MIC Range: Colistin: 0.25 µg/mL to 16 µg/mL, Piperacillin-tazobactam: 0.5/4 µg/mL to 64/4 µg/mL
- Application: Antimicrobial susceptibility testing of Gram-negative organisms such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae (e.g., Escherichia coli and Klebsiella pneumoniae).
- Quantity: 4 tests per kit
- Interpretation: The MIC value is determined based on the lowest concentration of each drug where visible growth inhibition is observed.
Key Features:
- Dual antibiotic testing: Simultaneously determines Colistin and Piperacillin-tazobactam susceptibility.
- Precise MIC determination: Allows for accurate susceptibility profiling.
- Easy-to-read results: Clear inhibition zones that correlate with the MIC values for each antibiotic.
- Cost-effective: Each kit provides 4 tests, ensuring good value for routine testing in clinical laboratories.
- Flexible: Suitable for a wide range of Gram-negative pathogens, making it ideal for hospital or research use.
- Fast results: Results can be obtained in 16-24 hours.
Test Procedure:
- Preparation of the Bacterial Inoculum:
- Inoculate an isolated colony of the test organism into a sterile saline solution or Mueller-Hinton broth.
- Adjust the bacterial suspension to the 0.5 McFarland standard to ensure an accurate and standardized inoculum density.
- Inoculation of the Test Plate:
- Swab the inoculum evenly across the surface of Mueller-Hinton agar using a sterile swab.
- Allow the plate to dry for a few minutes to ensure the even distribution of bacteria.
- Placement of the MIC Test Strips:
- Place the ComASP™ MIC test strips for Colistin and Piperacillin-tazobactam on the surface of the inoculated agar plate.
- Ensure the strips are spaced at least 15 mm apart to prevent interference.
- Incubation:
- Incubate the plate aerobically at 37°C for 16–24 hours.
- Reading the Results:
- After incubation, measure the zone of inhibition around the strip and determine the MIC value.
- The MIC value corresponds to the lowest concentration where no visible growth is observed.
Interpretation of Results:
- Colistin:
- MIC ≤ 2 µg/mL: Susceptible
- MIC > 2 µg/mL: Resistant (may vary based on specific resistance breakpoints from EUCAST or CLSI guidelines)
- Piperacillin-tazobactam:
- MIC ≤ 16/4 µg/mL: Susceptible
- MIC > 16/4 µg/mL: Resistant
- Note: Consult the EUCAST or CLSI guidelines for exact resistance breakpoints for Colistin and Piperacillin-tazobactam based on the species tested.
Reference Data:
- EUCAST guidelines (European Committee on Antimicrobial Susceptibility Testing):
- Colistin resistance: Any MIC > 2 µg/mL for Pseudomonas and Acinetobacter.
- Piperacillin-tazobactam: MIC breakpoint ≥16 µg/mL for Pseudomonas aeruginosa.
- CLSI guidelines (Clinical and Laboratory Standards Institute):
- Colistin resistance: MIC > 2 µg/mL for Pseudomonas aeruginosa, Acinetobacter baumannii.
- Piperacillin-tazobactam resistance: MIC > 16/4 µg/mL for Enterobacteriaceae.
Comparisons with Other Brands:
- Liofilchem® ComASP™ vs. Oxoid™ and Mast Diagnostics:
- Dual antibiotic testing: Unlike many competitors, Liofilchem® ComASP™ strips allow for simultaneous testing of Colistin and Piperacillin-tazobactam, making it a more convenient solution for detecting resistance profiles in Gram-negative pathogens.
- Microscopy vs. strip tests: ComASP™ strips offer a precise MIC value compared to disc diffusion methods offered by brands like Oxoid™ or Mast Diagnostics, where zone diameters may be more prone to variability.
- Liofilchem® vs. Broth Microdilution:
- Broth microdilution methods can be labor-intensive and require more time to prepare dilutions, whereas the ComASP™ strips provide a quicker, easier alternative for MIC testing.
- Liofilchem® vs. PCR-based Testing:
- PCR-based tests can detect resistance genes but are often costly and complex. Liofilchem® MIC strips are a cost-effective, rapid solution for detecting Colistin and Piperacillin-tazobactam resistance directly in clinical samples.
Conclusion:
The Liofilchem® ComASP™ Colistin / Piperacillin-tazobactam MIC Test (4 tests) offers a reliable, simple, and fast method for detecting antimicrobial resistance in Gram-negative bacteria. By simultaneously testing Colistin and Piperacillin-tazobactam, the test provides essential information for clinicians when treating multidrug-resistant infections. The easy-to-read MIC results and fast turnaround time make this test a valuable tool for routine resistance screening in clinical microbiology laboratories. When compared to other brands, Liofilchem® offers superior ease of use, cost-efficiency, and reliability for combination antibiotic testing.




 0
0