AutoMic AST i600
Catalog No :
CAS Number :
Brand :
In Stock
Automated Microbial Identification and Antibiotic Susceptibility Testing (ID/AST) System
Specifications:
| Application | Microbial Identification/AST | Warranty | 1 Year |
| Storage Temperature | Ambient | ||
| Product Type | Laboratory Equipment | ||
| Product Brand | Autobio Diagnostics | ||
| Product Grade | Microbiology grade | ||
Automated Microbial Identification and Antibiotic Susceptibility Testing (ID/AST) System
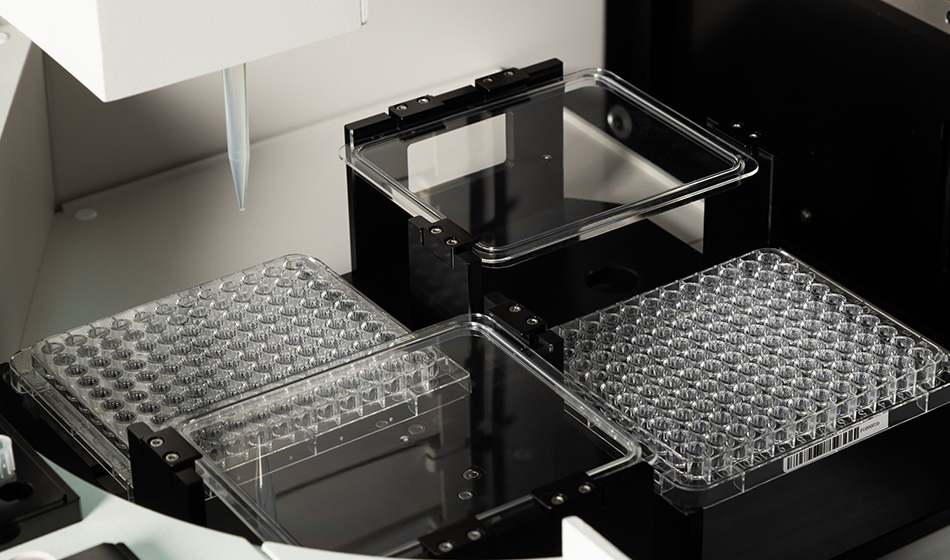
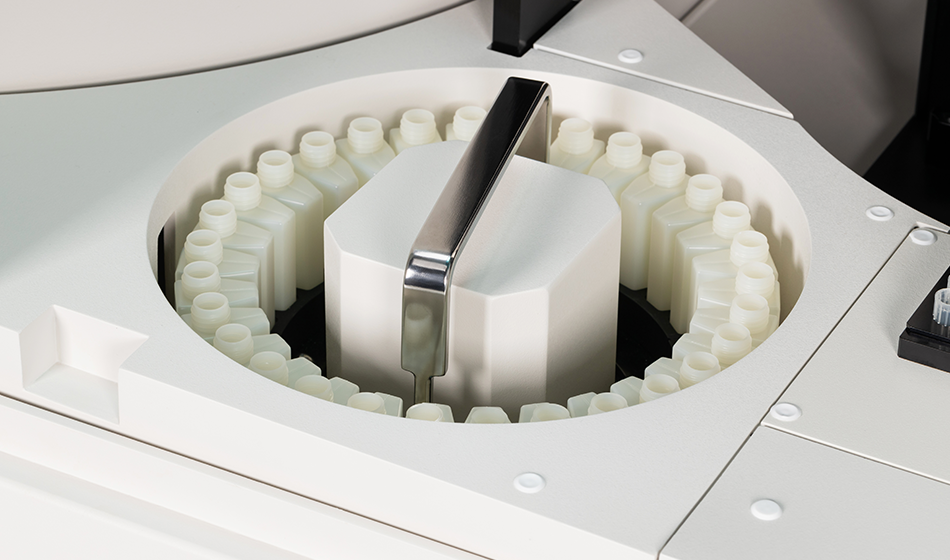
The Autobio AutoMic AST i600 is an advanced automated system designed for rapid microbial identification (ID) and antibiotic susceptibility testing (AST) based on the broth microdilution reference method.
Combining turbidimetric and colorimetric detection with a comprehensive expert software platform, the i600 provides accurate, reliable, and real-time antimicrobial resistance profiling for clinical microbiology laboratories.
The system supports automated sample pipetting, incubation, and detection for true minimal inhibitory concentration (MIC) determination—helping clinicians make faster, evidence-based therapeutic decisions.
Key Features
Hardware Features
- Broth microdilution reference method: Gold standard for MIC determination using turbidimetry and colorimetry.
- Fully automated workflow: Integrated sample scanning, inoculation, incubation, and result interpretation.
- Smart card recognition: Bidirectional intelligent identification and automatic matching of cards and specimens.
- Flexible card management: Supports random placement of multiple AST and ID card types.
-
High-capacity design:
- 120-well card for high throughput.
- 64-card incubator for simultaneous testing.
- Accurate and real-time detection: Delivers precise MIC values through continuous monitoring.
Software Features
- Customizable reporting: Create and export tailor-made result templates for hospital or laboratory systems.
- Barcode-based paperless management: Two-way sample and card identification ensures full traceability.
- Comprehensive strain database: Developed jointly by multiple reference centers for reliable microbial identification.
- Expert system integration: Based on the latest CLSI and EUCAST standards with continuous update services.
- Automated resistance interpretation: Detects and adjusts for resistance mechanisms such as ESBLs, CRE, MRSA, VRE, and PRSP.
- Direct WHONET data export: Facilitates global surveillance reporting and antimicrobial stewardship.
Technical Specifications
| Parameter | Specification |
|---|---|
| Maximum Capacity | 64 cards |
| Sample Injection Method | Automatic sample addition |
| Speed | <3 min / 120 samples |
| Pipetting Accuracy | 50 µL ±3 µL, 100 µL ±3 µL, 150 µL ±3 µL |
| Pipetting Repeatability | CV <3% |
| Incubation Temperature | 35°C ±1.5°C |
| Wavelength Range | 400–700 nm |
| Light Source | LED |
| Number of Filters | Max 6 |
Affiliated AST Cards for i600
| No. | Card Name | Number of Antibiotics | Range of Application | Advantages |
|---|---|---|---|---|
| 1 | Gram-positive, GP | 20 | Staphylococcus, Enterococcus, Bacillus | 20 MIC tests + Horizontal streptomycin resistance test; includes Oritavancin, Ceftaroline, Daptomycin. |
| 2 | Enterobacteriaceae, EB | 26 | Enterobacteria | ESBL confirmatory test; key drugs: Ceftazidime/Avibactam, Moxifloxacin, Tigecycline. |
| 3 | Non-fermenters, NF | 22 | Pseudomonas, Acinetobacter, Burkholderia, Stenotrophomonas | Tests: Piperacillin/Tazobactam, Polymyxin B, Tigecycline, Ceftazidime/Avibactam. |
| 4 | Streptococcus, ST | 19 | Streptococcus | 19 MIC tests + Clindamycin Induced Resistance Test. |
| 5 | Fungal, FG | 10 | Candida, Cryptococcus, Aspergillus | 10–14 antifungal concentration gradients, quantitative detection. |
Applications
- Clinical Microbiology: Identification and AST for bacterial and fungal pathogens.
- Hospital & Reference Labs: Supports antimicrobial resistance surveillance and stewardship.
- Pharmaceutical Research: Drug discovery and antibiotic efficacy testing.
- Food and Water Microbiology: Rapid detection of resistant organisms in quality assurance workflows.
- Public Health Laboratories: Data integration for WHONET and national AMR reporting.
Unique Advantages
- High-throughput, fully automated operation reduces manual handling.
- Accurate, reference-standard MIC determination using broth microdilution.
- Comprehensive antibiotic panels covering Gram-positive, Gram-negative, fungal, and streptococcal species.
- Upgradable expert software with ongoing CLSI/EUCAST updates.
- Direct data export to WHONET for antimicrobial resistance reporting.
- Compact design with intelligent temperature control and multi-card capacity.
The Autobio AutoMic AST i600 is a state-of-the-art automated ID/AST platform combining precision broth microdilution, AI-driven resistance interpretation, and real-time MIC detection.
Supporting both bacterial and fungal susceptibility testing, it enables microbiology laboratories to deliver accurate, reproducible, and timely antimicrobial results, essential for infection control, patient management, and AMR surveillance.




 0
0