Specifications:
| Application | qPCR | ||
| Storage Temperature | -20°C | ||
| Product Type | PCR Reagent | Forms | Liquid |
| Product Brand | Altona Diagnostics | ||
| Product Grade | Molecular Biology | ||
CE-IVD Certified Real-Time PCR Test for Dengue Virus RNA Detection
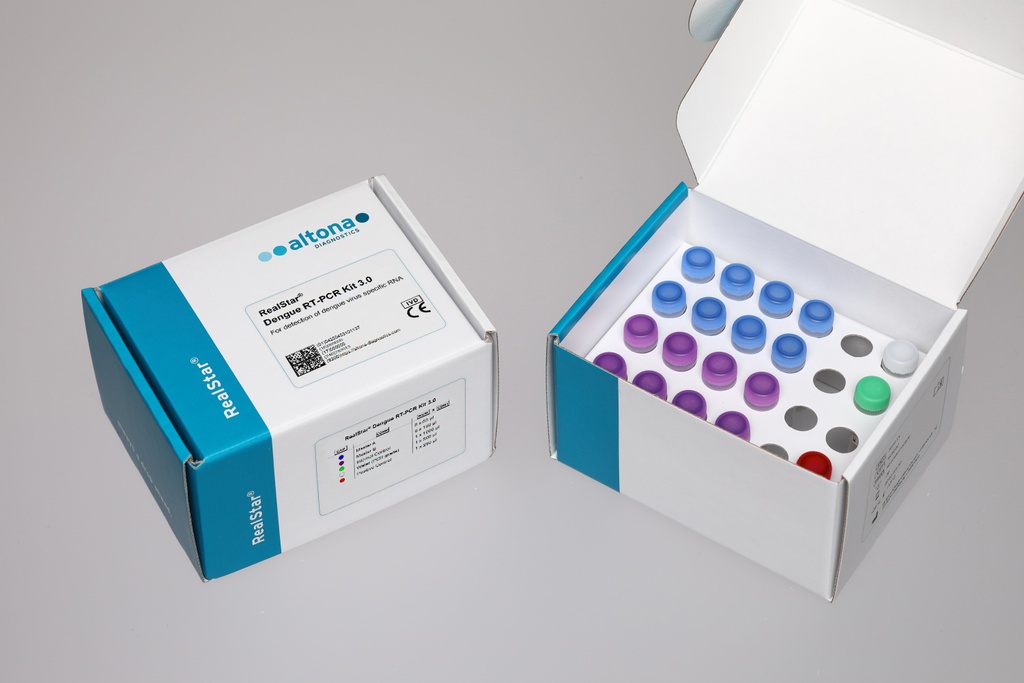
The RealStar® Dengue RT-PCR Kit 3.0 is an in vitro diagnostic (IVD) real-time PCR assay designed for the qualitative detection of dengue virus RNA. This CE-IVD marked test ensures accurate and reliable detection of all four dengue virus serotypes (DENV 1–4), making it an essential tool for clinical diagnostics and epidemiological surveillance.
Compatible with multiple real-time PCR platforms, this ready-to-use kit includes an Internal Control and Positive Control, ensuring reproducible and high-sensitivity results.
Key Features & Benefits
✅ Qualitative Detection of Dengue Virus RNA – Targets all four serotypes (DENV 1–4)
✅ Ready-to-Use Kit – Includes Internal Control (IC) and Positive Control (PC) for assay validation
✅ Compatible with Multiple PCR Platforms – Works with leading real-time PCR instruments
✅ CE-IVD Certified – Meets regulatory standards for clinical diagnostic use
✅ Highly Sensitive & Specific – Uses real-time PCR technology for precise dengue RNA detection
✅ Reliable & Reproducible Results – Ensures accurate patient diagnosis and disease monitoring
Technical Specifications
| Attribute | Details |
|---|---|
| Product Name | RealStar® Dengue RT-PCR Kit 3.0 |
| Intended Use | Qualitative detection of dengue virus RNA (DENV 1–4) |
| Kit Components | Internal Control (IC), Positive Control (PC), PCR Master Mix |
| Regulatory Status | CE-IVD Certified |
| Number of Reactions | 96 Rxns |
| Detection Method | Real-Time PCR (RT-PCR) Technology |
| Storage Conditions | Shipped on dry ice, store as recommended in protocol |
| Transportation Conditions | Dry Ice |
| Order Number | 283013 |
Compatible Real-Time PCR Platforms
✔ Mx 3005P™ QPCR System (Stratagene)
✔ VERSANT® kPCR Molecular System AD (Siemens Healthcare)
✔ ABI Prism® 7500 SDS (Applied Biosystems)
✔ ABI Prism® 7500 Fast SDS (Applied Biosystems)
✔ Rotor-Gene® 6000 (Corbett Research)
✔ Rotor-Gene® Q5/6 Plex Platform (QIAGEN)
✔ CFX96™ Real-Time PCR Detection System (Bio-Rad)
✔ CFX96™ Deep Well Real-Time PCR Detection System (Bio-Rad)
✔ LightCycler® 480 Instrument II (Roche)
Applications
🔬 Clinical Diagnostics – Used in hospitals, diagnostic labs, and reference laboratories
🦠 Epidemiological Surveillance – Helps track dengue outbreaks and virus prevalence
🧪 Infectious Disease Research – Supports molecular studies on dengue virus pathogenesis
⚕ Public Health Programs – Assists in vector-borne disease monitoring and prevention strategies
🛠 Pharmaceutical & Vaccine Development – Aids in dengue vaccine efficacy studies
Usage Guidelines
1️⃣ Sample Collection & Preparation – Extract RNA from patient serum or plasma samples.
2️⃣ PCR Reaction Setup – Add template RNA, PCR Master Mix, and Internal Control (IC).
3️⃣ Real-Time PCR Amplification – Run the reaction on compatible thermal cyclers.
4️⃣ Result Interpretation – Fluorescence-based detection determines dengue virus presence.
5️⃣ Quality Control Check – Validate results using the Positive Control (PC) and Internal Control (IC).
Regulatory & Safety Information
⚠️ For In Vitro Diagnostic (IVD) Use Only – Not intended for research or experimental applications
⚠️ CE-IVD Marked – Complies with European clinical diagnostic standards
⚠️ Biosafety Precautions Required – Handle patient samples under Biosafety Level 2 (BSL-2) or higher
Why Choose the RealStar® Dengue RT-PCR Kit 3.0?
🔹 Comprehensive Detection of Dengue Virus (DENV 1–4) – Ensures accurate differentiation of all four serotypes
🔹 Validated for Clinical & Diagnostic Use (CE-IVD Certified) – Meets international regulatory standards
🔹 High Sensitivity & Specificity with Real-Time PCR – Provides reliable detection of low viral loads
🔹 Compatible with Leading PCR Platforms – Works seamlessly with Bio-Rad, Roche, QIAGEN, Applied Biosystems & Siemens systems
🔹 Complete Ready-to-Use Kit with Internal Controls – Ensures assay integrity and accuracy
For bulk orders, technical support, or additional information, contact LabMart Limited today.




 0
0