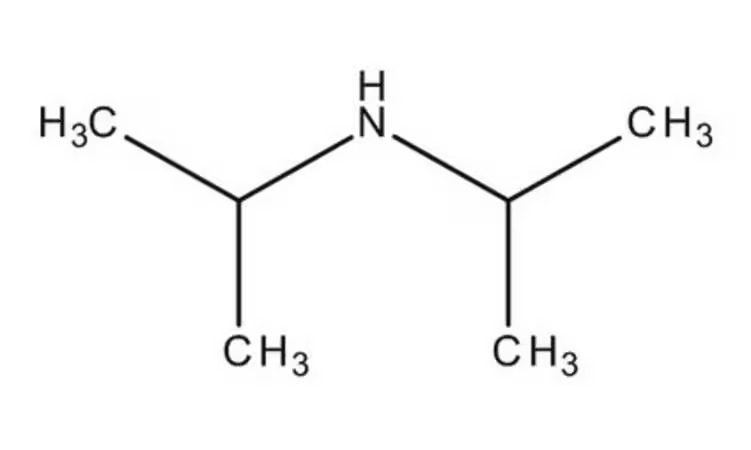
Specifications:
| Application | Organic Synthesis | ||
| Storage Temperature | Ambient | ||
| Product Type | Laboratory Chemical | Forms | Liquid |
| Product Brand | Thermo Fisher Scientific™ | ||
| Product Grade | Analytical grade | Formula | C₆H₁₅N |
Specifications:
| Feature | Test Results |
|---|---|
| Appearance | Clear liquid |
| GC Purity | ≥99.0% |
| Refractive Index (20°C) | 1.3910 to 1.3930 (589 nm) |
| Water Content | ≤0.2% |
| Color Scale | ≤10 APHA |
| Infrared Spectrum | Conforms |
Description:
Diisopropylamine (DIPA) is an aliphatic secondary amine with a 99+% purity that is widely used in organic synthesis and chemical reactions. As a strong base, it is commonly utilized in deprotonation reactions, cross-coupling reactions, and the synthesis of various organic compounds. Diisopropylamine is an essential reagent for Pd-catalyzed reactions such as the Heck and Sonogashira reactions, which are used in the formation of carbon-carbon bonds.
Additionally, DIPA is a precursor in the preparation of lithium diisopropylamide (LDA), a strong non-nucleophilic base used extensively in organic synthesis. The product is also used as a catalyst in the oxidative coupling of aryl thiols and for the synthesis of furan derivatives.
Key Features:
-
High Purity (99%):
- This diisopropylamine has ≥99.0% purity, ensuring consistent performance in chemical synthesis, organic reactions, and analytical applications.
-
Versatile Base:
- Acts as a base in cross-coupling reactions, asymmetric syntheses, and the formation of non-nucleophilic bases like LDA.
-
Reagent for Organic Synthesis:
- Widely used for synthetic applications such as deprotonation, carbon-carbon bond formation, and functional group transformations.
-
High Reactivity:
- Its strong basic properties make it highly effective in organic chemistry, particularly in Pd-catalyzed and organometallic reactions.
-
Versatile Applications:
- Employed in the synthesis of furan, dihydrofuran, butanolide derivatives, and aryl disulfides via oxidative coupling reactions.
Applications:
-
Cross-Coupling Reactions:
- Used in Pd-catalyzed cross-coupling reactions such as the Heck and Sonogashira reactions, facilitating the formation of carbon-carbon bonds in organic synthesis.
-
Synthesis of Aryl Disulfides:
- Catalyst in the oxidative coupling of aryl thiols to form aryl disulfides in the presence of air as an oxidant.
-
Synthesis of Lithium Diisopropylamide (LDA):
- Diisopropylamine is used as a precursor to LDA, a strong base used in deprotonation reactions in organic chemistry.
-
Synthesis of Furan and Butanolide Derivatives:
- Diisopropylamine reacts with various substrates to synthesize furan derivatives and butanolides, compounds important in biochemical and industrial chemistry.
-
Pharmaceutical and Fine Chemical Synthesis:
- Used in the synthesis of pharmaceutical intermediates, fine chemicals, and specialty compounds in pharmaceutical and chemical industries.
Packaging Options:
| Catalog Number | Pack Size | Packaging |
|---|---|---|
|
219432500
| 250 mL | Glass bottle |
| 219430010 | 1L | Glass bottle |
Storage:
- Store at ambient temperature in a cool, dry place to maintain purity and reactivity.
Thermo Fisher Scientific Diisopropylamine 99+% is a versatile reagent widely used in organic synthesis, particularly in cross-coupling reactions, deprotonation reactions, and the synthesis of pharmaceutical intermediates. Its high purity, strong basic properties, and effective reactivity make it an essential reagent for both chemical synthesis and biochemical research.
- Pack Size: 1L 250 mL




 0
0