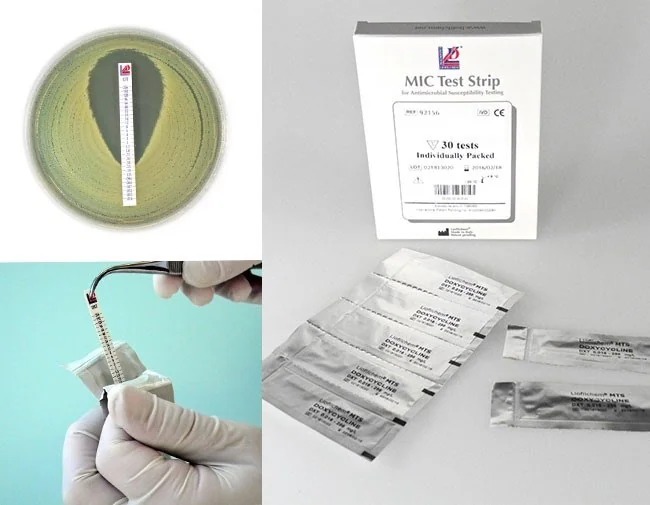
Liofilchem™ MTS™ Ampicillin-sulbactam SAM 0.016/4-256/4, MIC Test strips
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | MIC Strips |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem™ MTS™ Ampicillin-Sulbactam (SAM) MIC Test Strips are advanced antimicrobial gradient diffusion tools used to determine the Minimum Inhibitory Concentration (MIC) of ampicillin-sulbactam, a beta-lactam and beta-lactamase inhibitor combination. These strips are specifically designed to assess the susceptibility of bacterial pathogens to the combination of ampicillin and sulbactam in a 2:1 ratio, supporting precise and reliable antimicrobial susceptibility testing (AST).
Key Features
- Dual-Drug Combination: Test strips impregnated with a gradient concentration of ampicillin and sulbactam in a fixed 2:1 ratio.
- MIC Range: Detects MIC values from 0.016/4 µg/mL to 256/4 µg/mL, suitable for a wide variety of clinical pathogens.
- High Precision: Ensures accurate determination of the MIC values, supporting clinical decisions for treatment of infections caused by beta-lactamase-producing bacteria.
- Easy-to-Use: Designed for seamless application in clinical microbiology laboratories with results interpretable as a precise MIC value.
- Breakpoints Compatible: Results are compatible with CLSI and EUCAST guidelines for antimicrobial susceptibility testing.
Applications
- Clinical Microbiology:
- Determining susceptibility of Gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp..
- Testing against beta-lactamase-producing bacteria that are resistant to standalone beta-lactams.
- Antimicrobial Resistance Monitoring:
- Supporting surveillance programs to track resistance patterns in hospital and community-acquired infections.
- Research and Development:
- Used in pharmaceutical studies for evaluating combination therapies involving ampicillin-sulbactam.
Test Procedure
- Preparation:
- Prepare Mueller-Hinton agar (MHA) or another suitable medium following standard protocols.
- Adjust the bacterial suspension to 0.5 McFarland standard.
- Application:
- Apply the MTS™ Ampicillin-Sulbactam strip onto the inoculated agar surface using sterile forceps.
- Ensure complete contact between the strip and agar.
- Incubation:
- Incubate plates at 35 ± 2°C in an aerobic environment for 16-20 hours.
- Result Interpretation:
- Observe the intersection of the inhibition ellipse with the MIC scale on the strip.
- Report results as the MIC of the ampicillin-sulbactam combination in µg/mL.
Quality Control
The performance of MTS™ Ampicillin-Sulbactam strips is validated using the following control organisms:
- Escherichia coli ATCC® 25922 – Expected MIC within quality control ranges.
- Klebsiella pneumoniae ATCC® 700603 – Beta-lactamase positive control.
- Pseudomonas aeruginosa ATCC® 27853 – Beta-lactamase negative control.
Storage and Stability
- Store at 2–8°C in a dry environment, protected from light.
- Use before the expiration date printed on the packaging.
Ordering Information
| Product | Format | Catalog Number |
|---|---|---|
| MTS™ Ampicillin-sulbactam SAM 0.016/4-256/4 | 30 strips per pack | 92181 |
MTS™ Ampicillin-sulbactam SAM 0.016/4-256/4 | 10 Strips per pack |
921811
|
| MTS™ Ampicillin-sulbactam SAM 0.016/4-256/4 | 100 strips per pack | 921810 |
Precautions
- For in vitro diagnostic use (IVD) only.
- Intended for use by trained laboratory professionals.
- Ensure proper disposal of used strips and culture plates in accordance with institutional and local biosafety guidelines.
Regulatory Compliance
- Meets the standards set by CLSI, EUCAST, and ISO 11133 for quality control in antimicrobial susceptibility testing.
References
- Clinical and Laboratory Standards Institute (CLSI) guidelines.
- EUCAST breakpoint interpretations.
- Manufacturer’s technical documentation for Liofilchem MTS™ strips.
Key Benefits
- Comprehensive testing for beta-lactam/beta-lactamase inhibitor resistance.
- Simple and reliable methodology for MIC determination.
- Supports rapid and informed clinical decision-making in antimicrobial therapy.
- Pack Size: 100/pk 10/pk 30/pk




 0
0