Liofilchem® KPC & MBL Disc Kit, (acc. to EUCAST) for 50 Tests
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Clinical microbiology |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Liofilchem® KPC&MBL Disc Kit is a diagnostic tool designed for the confirmation of carbapenemase-producing Enterobacteriaceae (CRE), according to EUCAST guidelines. Below are the key highlights about its usage and purpose:
Purpose
- To confirm the presence of carbapenemases in Enterobacteriaceae suspected of carbapenem resistance.
- Differentiates between:
- Klebsiella pneumoniae carbapenemases (KPC) - Class A.
- Metallo-β-lactamases (MBL) - Class B (e.g., VIM, IMP, NDM).
- Other mechanisms like AmpC overproduction with porin loss.
Key Features
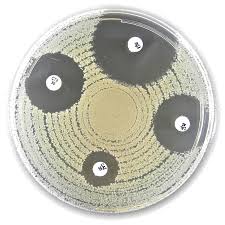
- Disc Synergy Testing:
- Uses meropenem discs alone and in combination with specific inhibitors:
- Phenylboronic acid: Inhibits KPC (Class A β-lactamases).
- EDTA: Inhibits MBL (Class B β-lactamases).
- Cloxacillin: Differentiates AmpC β-lactamases combined with porin loss.
- No inhibitor exists for Class D OXA-48-like enzymes, so its presence is inferred by exclusion.
- Uses meropenem discs alone and in combination with specific inhibitors:
- Test Procedure:
- Prepare bacterial suspensions (0.5 McFarland standard).
- Inoculate Mueller-Hinton agar plates.
- Place discs (meropenem alone and combinations).
- Incubate at 36 ± 1°C for 18–24 hours.
- Measure and compare inhibition zones to confirm carbapenemase types.
- Interpretation:
- Based on zone differences (≥4 mm with inhibitors indicates specific resistance mechanisms).
- Quality Control:
- QC performed with reference strains like Klebsiella pneumoniae (ATCC 700603 for negative control, BAA-2146 for MBL-positive, etc.).
Applications
- Helps in the phenotypic confirmation of carbapenemases in clinical isolates.
- Critical for choosing appropriate antimicrobial therapy.
- Supports infection control efforts by identifying and tracking resistance mechanisms.
Limitations
- The test does not directly identify Class D carbapenemases (e.g., OXA-48).
- In vitro conditions cannot completely replicate in vivo complexity.
- Influenced by variables like incubation conditions, medium quality, and disc placement.
The Liofilchem® KPC&MBL Disc Kit is designed for the phenotypic confirmation of carbapenemase-producing Enterobacteriaceae, following EUCAST guidelines. The kit includes the following components:
- Meropenem (MRP) 10 µg Disc: Serves as the baseline for assessing carbapenem susceptibility.
- Meropenem 10 µg + Phenylboronic Acid (MR+BO) Disc: Detects class A carbapenemases (e.g., KPC) by inhibiting serine β-lactamases.
- Meropenem 10 µg + EDTA (MR+ED) Disc: Identifies class B metallo-β-lactamases (e.g., VIM, IMP, NDM) through metal ion chelation.
- Meropenem 10 µg + Cloxacillin (MR+CL) Disc: Differentiates AmpC β-lactamase production combined with porin loss by inhibiting AmpC enzymes.
Each component is provided in cartridges containing 50 discs, individually packaged with a desiccant to maintain integrity.
References:
- EUCAST Guidelines: "EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance," Version 1.0, 2013.
- Giske et al. (2011): "A sensitive and specific assay for detection of MBL and KPC in K. pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin," Clinical Microbiology and Infection, 17, 552-556.
- Tsakris et al. (2010): "A simple phenotypic method for the differentiation of MBL and Class A KPC carbapenemase in Enterobacteriaceae clinical isolates," Journal of Antimicrobial Chemotherapy, 65, 1664-1671.
These references provide detailed methodologies and validation studies pertinent to the use of the KPC&MBL Disc Kit in clinical microbiology.
This kit is an essential component of microbiological diagnostics to address the growing challenge of carbapenem resistance in Enterobacteriaceae.




 0
0