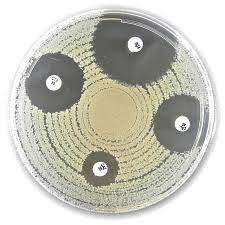
Liofilchem® Rifampicin R Antimicrobial Susceptibility Discs, 5 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Standardized Disc for Rifampicin Susceptibility Testing in Bacterial Cultures
The Liofilchem® Rifampicin R Antimicrobial Susceptibility Discs (5 µg) are standardized antibiotic discs designed for quantitative antimicrobial susceptibility testing (AST) using the Kirby-Bauer disk diffusion method. These discs are used to determine bacterial resistance or susceptibility to Rifampicin (RIF), a broad-spectrum RNA polymerase inhibitor primarily used against Mycobacterium tuberculosis, Staphylococcus spp., and Neisseria meningitidis.
These discs comply with CLSI (Clinical and Laboratory Standards Institute) and EUCAST (European Committee on Antimicrobial Susceptibility Testing) standards, ensuring accuracy and reproducibility in clinical microbiology laboratories, hospital infection control units, and research institutions.
Key Features & Benefits
✅ Standardized 5 µg Rifampicin Discs – Ensures consistent & reliable susceptibility testing
✅ CLSI & EUCAST Compliant – Meets international antimicrobial testing guidelines
✅ Highly Effective Against Gram-Positive Bacteria – Particularly used for Mycobacterium tuberculosis & Staphylococcus spp.
✅ Ready-to-Use Format – Pre-impregnated antibiotic discs allow direct application onto agar plates
✅ Long Shelf Life & Stability – Manufactured for extended use with proper storage
✅ Ideal for Kirby-Bauer Disk Diffusion Method – Provides accurate zone diameter measurement for AST
✅ Essential for Clinical & Research Microbiology – Helps in guiding effective antibiotic therapy
Technical Specifications
| Attribute | Details |
|---|---|
| Product Name | Liofilchem® Rifampicin R Antimicrobial Susceptibility Discs |
| Concentration | 5 µg |
| Active Ingredient | Rifampicin (RIF) |
| Disc Diameter | 6 mm |
| Testing Method | Kirby-Bauer Disc Diffusion |
| Bacterial Targets | Gram-positive bacteria, Mycobacterium spp., and select Gram-negative bacteria |
| Storage Conditions | 2–8°C in a dry environment |
| Regulatory Compliance | CLSI & EUCAST Standards |
| Format | Cartridge with multiple discs |
| Application | Antimicrobial susceptibility testing for Rifampicin |
Use Cases & Applications
1. Mycobacterium tuberculosis Susceptibility Testing
🔬 Clinical Microbiology & TB Reference Labs
- Determines resistance patterns in Mycobacterium tuberculosis isolates.
- Guides first-line anti-TB treatment decisions.
- Used alongside other first-line TB antibiotics (Isoniazid, Ethambutol, Pyrazinamide).
2. Staphylococcus aureus & MRSA Testing
🦠 Hospital Infection Control & Clinical Labs
- Detects Rifampicin resistance in Staphylococcus aureus & MRSA strains.
- Supports effective treatment strategies for Rifampicin-based combination therapy.
3. Neisseria meningitidis & Haemophilus influenzae Testing
⚗️ Bacteriology Labs & Public Health Surveillance
- Used for meningococcal & respiratory tract pathogen susceptibility analysis.
- Essential for outbreak management & prophylaxis selection.
4. Multi-Drug Resistance (MDR) Surveillance
🏥 Epidemiological & Research Studies
- Monitors emerging Rifampicin-resistant bacterial strains.
- Supports antimicrobial stewardship programs & global health initiatives.
How to Use the Rifampicin R 5 µg Discs in the Kirby-Bauer Method
1️⃣ Prepare a standardized bacterial inoculum (0.5 McFarland standard).
2️⃣ Evenly spread the bacterial suspension onto Mueller-Hinton agar plates.
3️⃣ Place Rifampicin R 5 µg discs onto the agar surface with sterile forceps or an automatic disc dispenser.
4️⃣ Incubate at 35 ± 2°C for 16–20 hours (conditions vary for different bacteria).
5️⃣ Measure the zone of inhibition around the disc using a calibrated ruler or automated zone reader.
6️⃣ Compare results with CLSI or EUCAST breakpoints to determine susceptibility or resistance.
Interpretation of Results (CLSI/EUCAST Guidelines)
| Bacterial Species | Susceptible (S) Zone Diameter (≥ mm) | Resistant (R) Zone Diameter (≤ mm) |
|---|---|---|
| Mycobacterium tuberculosis | ≥20 mm | ≤19 mm |
| Staphylococcus aureus (MSSA/MRSA) | ≥20 mm | ≤19 mm |
| Neisseria meningitidis | ≥25 mm | ≤24 mm |
(Values may be updated based on CLSI/EUCAST guidelines, always refer to the latest breakpoint tables for accurate interpretation.)
Ordering Information
| Pack Size | Catalog Number |
|---|---|
| 50 Discs per Cartridge | 9118 |
Storage & Handling
- Store at 2–8°C in a dry, contamination-free environment.
- Do not expose discs to moisture or excessive heat to maintain potency.
- Use aseptic techniques when handling discs to prevent contamination.
- Check expiration date before use to ensure reliable results.
Regulatory & Safety Information
⚠️ For In Vitro Diagnostic (IVD) Use Only. Not for human or animal therapeutic applications.
⚠️ Handle Biosafety Level 2 (BSL-2) Pathogens with Care – Use proper biosafety precautions when testing bacterial cultures.
⚠️ Follow CLSI & EUCAST Guidelines – Ensure standardized antibiotic susceptibility testing methods.
Why Choose Liofilchem® Rifampicin R Antimicrobial Susceptibility Discs (5 µg)?
🔹 CLSI & EUCAST Compliant for Reliable AST Results
🔹 Standardized 5 µg Rifampicin Disc for Consistent Testing
🔹 Effective for TB, Staphylococcus, & Meningococcal Infections
🔹 Ideal for Hospital, Research, & Public Health Laboratories
🔹 Ready-to-Use Format for Convenient & Accurate Testing
For bulk orders, technical support, or additional information, contact LabMart Limited today. 🚀




 0
0