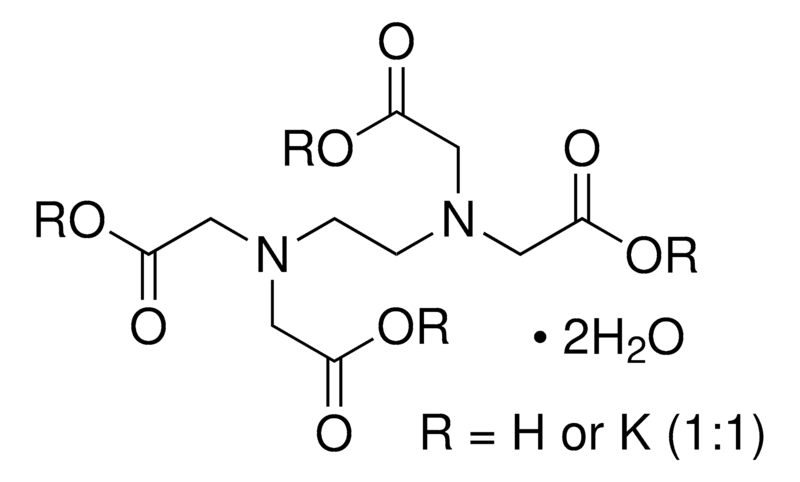
VWR EDTA dipotassium salt dihydrate ≥97.0% analytical reagent
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Storage Temperature | Ambient | ||
| Product Type | Laboratory Chemical | Forms | Powder |
| Product Brand | VWR | ||
| Product Grade | Analytical grade | Formula | C₁₀H₁₄K₂N₂O₈·2H₂O |
VWR EDTA Dipotassium Salt Dihydrate, ≥97.0%, is a high-purity chelating compound used to bind divalent and trivalent metal ions in analytical chemistry, complexometric titrations, biochemical buffer systems, and industrial applications. Its potassium salt form offers excellent solubility in water and is especially useful in formulations where sodium is not preferred.
Key Features
- ≥97% Purity – Reliable performance for analytical and laboratory applications
- Dipotassium Salt Form – Enhanced solubility in aqueous solutions
- Stable, Non-Hygroscopic Solid – Easy to handle and store
- Effective Metal Chelator – Strong complexing agent for Ca²⁺, Mg²⁺, Fe³⁺, and more
- Broad Compatibility – Suitable for use in biochemical buffers and reagent formulations
Specifications
| Property | Value |
|---|---|
| Product Name | EDTA Dipotassium Salt Dihydrate |
| CAS Number | 25102-12-9 |
| Chemical Formula | C₁₀H₁₄K₂N₂O₈·2H₂O |
| Molar Mass | 404.46 g/mol |
| Assay | ≥97.0% |
| Melting Point | 272 °C |
| Density (20 °C) | 1 g/cm³ |
| EINECS | 217-895-0 |
| Hazard Classification | Not classified as hazardous (GHS) |
| Storage Temperature | Ambient |
Applications
- Complexometric Titration – Precise determination of metal ion concentrations
- Biological & Biochemical Buffers – Key component in TE, TAE, and other buffer systems
- Analytical Chemistry – Metal ion masking in trace analysis
- Water Softening & Treatment – Chelates hardness ions in water conditioning
- Pharmaceutical & Cosmetic Formulations – Stabilizer against metal-catalyzed oxidation
Ordering Information
| Catalog Number | Pack Size | Packaging Type |
|---|---|---|
| 20490.188 | 100 g | Plastic bottle (solids) |
VWR® EDTA Dipotassium Salt Dihydrate, ≥97%, is a dependable and versatile chelating agent for laboratory titrations, buffer preparation, and industrial formulations where potassium ions are preferred over sodium.




 0
0