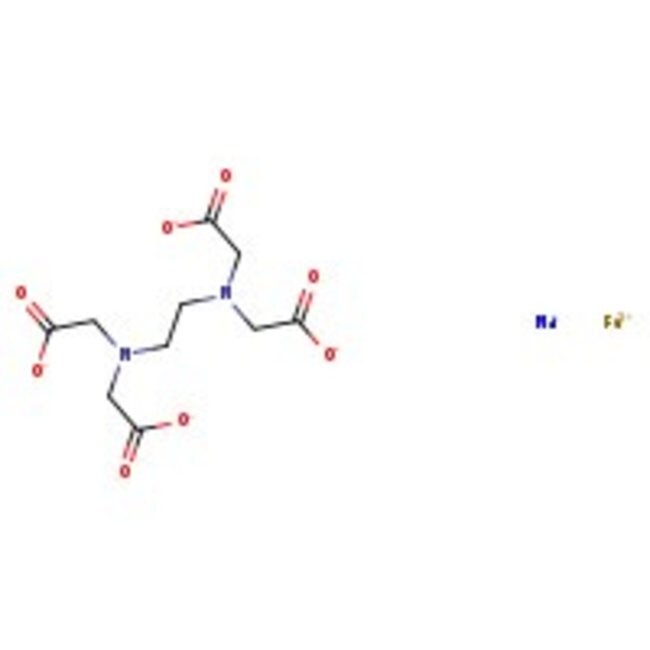
Sigma-Aldrich Sodium iron EDTA United States Pharmacopeia (USP) Reference Standard, 200mg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Quality Control | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Certified Reference Materials (CRMs) | Forms | Solid |
| Product Brand | Sigma-Aldrich | ||
| Product Grade | Analytical grade | Formula | C₁₀H₁₂FeN₂NaO₈·3H₂O |
Sodium Iron EDTA (Ethylenediaminetetraacetic acid iron(III) sodium salt hydrate) is supplied as a USP Reference Standard for pharmaceutical quality testing. With an empirical formula of C₁₀H₁₂FeN₂NaO₈·3H₂O and a molecular weight of 421.09 g/mol, this compound serves as a primary pharmaceutical standard for quality control and assay development in accordance with USP compendial methods.
It is provided in neat format and intended exclusively for analytical use in quality tests, assays, and compendial procedures.
Key Features
- USP Reference Standard – pharmaceutical primary standard for compendial testing.
- High purity – intended for use in validated quality control assays.
- Reliable performance – conforms to USP specifications for sodium iron EDTA.
- Standardized format – supplied as a neat reference material for assay reproducibility.
- Not for therapeutic use – strictly for laboratory testing and compendial quality evaluation.
Applications
- Reference material for USP assays and tests.
- Quality control in pharmaceutical R&D and production.
- Validation of analytical methods involving iron EDTA complexes.
- Standard for regulatory compliance in the pharmaceutical industry.
Technical Specifications
| Property | Value |
|---|---|
| Chemical Name | Sodium iron EDTA (USP) |
| Synonyms | Ethylenediaminetetraacetic acid iron(III) sodium salt hydrate |
| CAS Number | 18154-32-0 |
| Empirical Formula | C₁₀H₁₂FeN₂NaO₈·3H₂O |
| Molecular Weight | 421.09 g/mol |
| Form | Neat |
| Grade | Pharmaceutical primary standard (USP) |
| Storage | Store as directed by USP reference material handling |
| MDL Number | MFCD32221860 |
| UNSPSC Code | 41116107 |
| NACRES Code | NA.24 |
| Application | Pharmaceutical quality tests, assay validation |
Available Format
| Catalog Number | Quantity | Format |
|---|---|---|
| 1614239-200MG | 200 mg | USP Reference Standard |
Sodium Iron EDTA – USP Reference Standard provides laboratories with a certified primary standard for use in USP compendial tests and assays. It ensures accuracy, reproducibility, and compliance in pharmaceutical quality control and method validation, serving as an essential material for drug development and regulatory testing.




 0
0