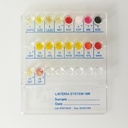
Specifications:
| Application | Microbial Identification/AST | ||
| Storage Temperature | 2-8°C | ||
| Product Type | Microbial ID System | Forms | Kit with Various components |
| Product Brand | Liofilchem | Target Organism Class | Listeria monocytogenes |
| Product Grade | Microbiology grade | ||
The Liofilchem® LISTERIA SYSTEM 18R is a robust and compact 18-well microbiological testing system designed for the biochemical identification of Listeria spp.. This system is a rapid and reliable tool for microbiology laboratories, enabling the presumptive identification of Listeria monocytogenes and related species from cultures grown on selective or non-selective agar media. Results are provided within 18-24 hours, with additional confirmatory tests to ensure precision and accuracy.
Key Features:
- Multi-Well Biochemical Identification:
- Detects and identifies various Listeria species through 18 biochemical tests:
- Listeria monocytogenes
- Listeria innocua
- Listeria grayi subsp. grayi
- Listeria grayi subsp. murrayi
- Listeria seeligeri
- Listeria welshimeri
- Listeria ivanovii
- Jonesia denitrificans
- Detects and identifies various Listeria species through 18 biochemical tests:
- Fast Results:
- Results are delivered in as little as 18-24 hours, ensuring rapid identification.
- Numerical Code Identification:
- Interpretation of results generates a 6-digit numerical code for precise identification, using predefined biochemical profiles.
- Validated and Reliable:
- Each system is validated for accuracy and subjected to rigorous quality control using reference strains.
- Ease of Use:
- Pre-configured wells with dried biochemical substrates simplify preparation and interpretation.
- Supplementary Confirmatory Tests:
- Includes additional tests, such as ABN, indole, catalase, methyl red, nitrate reduction, and VP tests for complete microbial identification.
Specifications:
| Attribute | Details |
|---|---|
| Catalog Number | 71640 |
| Detection Time | 18-24 hours |
| Number of Wells | 18 wells per system |
| Test Type | Biochemical identification of Listeria spp. |
| Applications | Food microbiology, clinical microbiology, environmental testing |
| Organisms Detected | Listeria spp. and Jonesia denitrificans |
| Storage Conditions | 2°C to 8°C |
| Tests per Kit | 20 systems (20 tests per kit) |
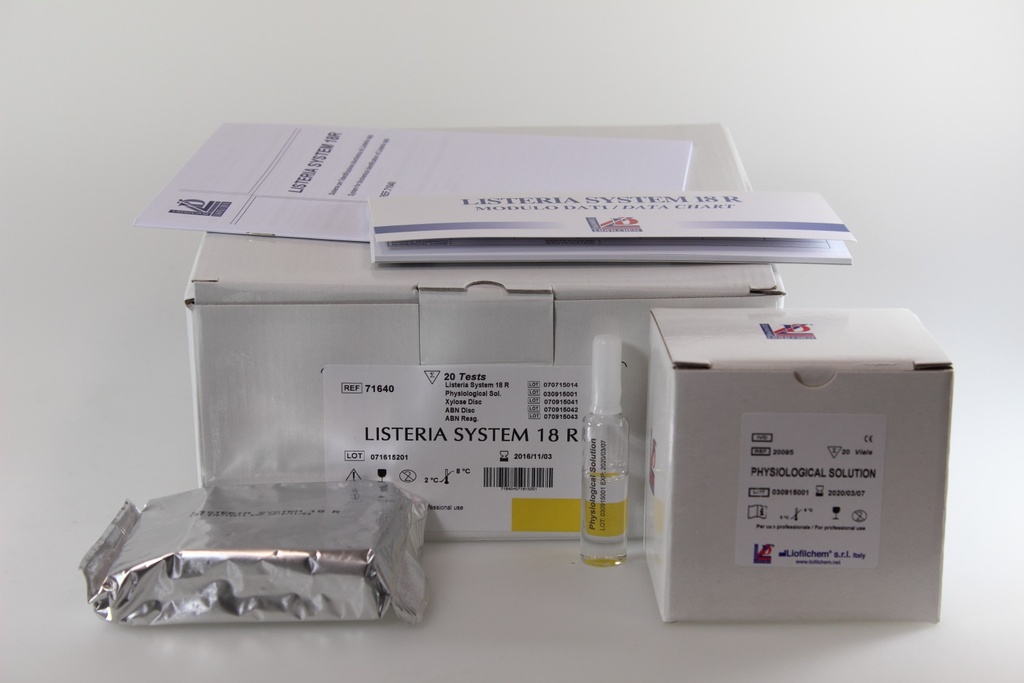
Kit Components:
- 20 LISTERIA SYSTEM 18R panels
- 20 Vials of Physiological Solution
- 20 Xylose Discs
- 20 ABN Discs
- 1 Vial of ABN Reagent (3 mL)
- Instruction Sheet
Additional Items Required (Not Included):
- LISTERIA SYSTEM 18R Reagent (Code 80257)
- Gram Color Kit (Code 80293)
- Vaseline Oil (Code 80278)
- Sundry laboratory materials (slides, cover slips, microscope).
Applications:
- Food Safety Testing:
- Identify Listeria spp. in food samples such as meat, dairy, and processed foods.
- Microbial Screening:
- Perform rapid and accurate biochemical identification of Listeria species from selective/non-selective agar cultures.
- Clinical and Environmental Microbiology:
- Screen samples from clinical or environmental sources for Listeria contamination.
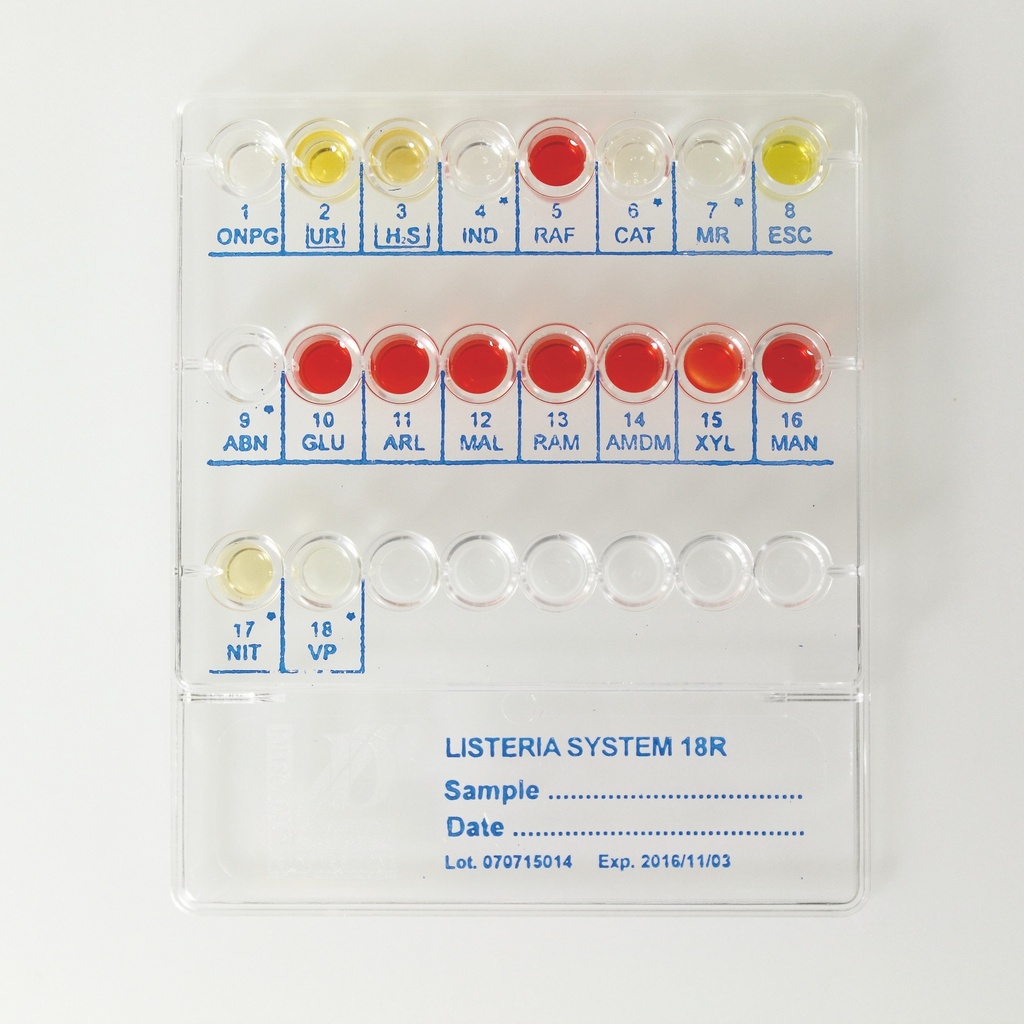
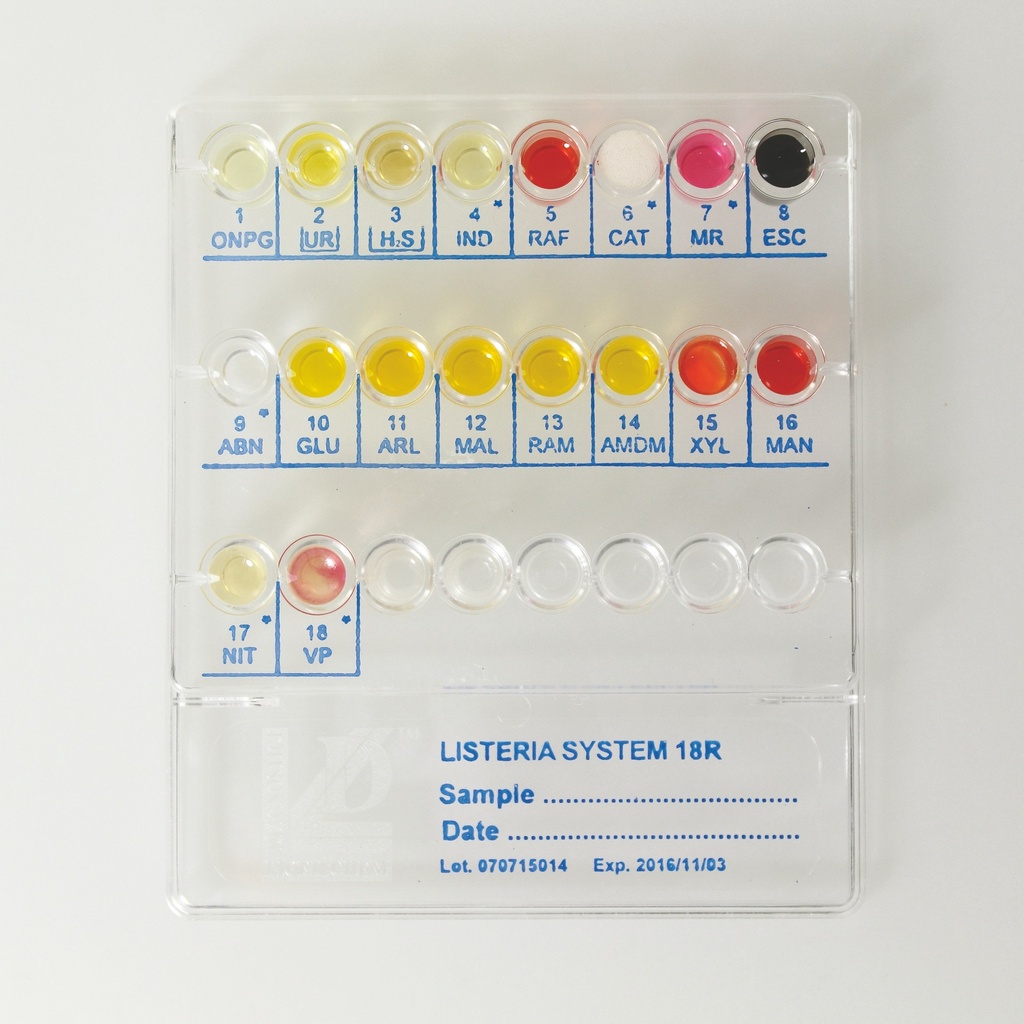
How It Works:
- Preparation of Bacterial Suspension:
- Observe colony morphology and confirm the sample belongs to the Listeria genus with Gram staining.
- Prepare a bacterial suspension using Physiological Solution provided in the kit.
- System Inoculation:
- Inoculate each well with 0.2 mL (4 drops) of the suspension.
- Add Xylose Disc to well 15 and cover wells 2 (UR) and 3 (H2S) with Vaseline Oil.
- Incubate at 36°C ± 1°C for 18-24 hours.
- Additional Tests:
- Conduct confirmatory tests, such as:
- ABN Test: Identifies non-monocytogenes species by observing yellow coloration in well 9-ABN.
- Indole Test: Add Kovac's reagent to well 4-IND.
- Catalase Test: Add H2O2 to well 6-CAT.
- Methyl Red Test: Add Methyl Red reagent to well 7-MR.
- Nitrate Test: Add Sulfanilic Acid and Naftilamine to well 17-NIT.
- VP Test: Add Alpha-Naphthol and NaOH 40% to well 18-VP.
- Conduct confirmatory tests, such as:
- Result Interpretation:
- Observe color changes in the wells and interpret using the included Numerical Code Table.
- Use the 6-digit numerical code to identify the organism from the Identification Table.
Key Benefits:
- Compact and Comprehensive:
- Performs 18 biochemical tests in a single panel, saving time and resources.
- Accurate and Reliable:
- Validated system with a high degree of accuracy for Listeria spp. identification.
- Flexible Testing:
- Supports additional biochemical and confirmatory testing for complete microbial profiling.
- Fast Results:
- Provides results in less than 24 hours, expediting decision-making in food safety and clinical diagnostics.
Validation and Quality Control:
- Validated against reference strains, including:
- Listeria monocytogenes (ATCC 35152)
- Listeria innocua (ATCC 33090)
- Listeria ivanovii (ATCC 19119)
- Quality control ensures reliable performance across batches.
Precautions:
- Ensure all materials are sterile and procedures are followed as per instructions.
- Use by trained personnel in a laboratory setting.
- Store at 2°C to 8°C and do not use expired systems.
Storage and Disposal:
- Store the kit at 2°C to 8°C in its original packaging.
- Dispose of used systems and associated materials following standard laboratory decontamination protocols.
References:
- McLannchlin, Identification of Listeria species, International Journal of Food Microbiology, 38 (1997) 77–81.
- Bailey and Scott’s Diagnostic Microbiology, 7th ed., C.V. Mosby Company, 1986.
- Edwin H. Lenette, Manual of Clinical Microbiology, 4th ed., ASM Washington, 1985.
For Research Use Only:
This product is intended for research purposes only and is not approved for diagnostic or therapeutic use.
- Pack Size: for 20 Tests for 4 Tests




 0
0